
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Molecular Switches
About this book
The long-awaited second edition of the successful book covering molecular switches now in two volumes!
Providing principles and applications this book brings you everything you need to know about molecular switches - a hot topic in the nanoworld. The major classes of molecular switches including catenanes, rotaxanes, azobenzenes together with polymer and biomolecular switching systems and DNA based switches are covered. Chemists and material scientists interested in one of the most innovative areas of their science will benefit greatly from reading this book.
"This book will appeal most to organic chemists, because of the way new structures are introduced through their synthesis, but it will also provide a useful introduction for other scientists, provided they are conversant with molecular structures." (Organic and Biomolecular Chemistry)
"... a comprehensive and up-to-date insight ..." (Chemistry & Industry)
Providing principles and applications this book brings you everything you need to know about molecular switches - a hot topic in the nanoworld. The major classes of molecular switches including catenanes, rotaxanes, azobenzenes together with polymer and biomolecular switching systems and DNA based switches are covered. Chemists and material scientists interested in one of the most innovative areas of their science will benefit greatly from reading this book.
"This book will appeal most to organic chemists, because of the way new structures are introduced through their synthesis, but it will also provide a useful introduction for other scientists, provided they are conversant with molecular structures." (Organic and Biomolecular Chemistry)
"... a comprehensive and up-to-date insight ..." (Chemistry & Industry)
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part I
MOLECULAR SWITCHING
1
Multifunctional Diarylethenes
1.1 Introduction
Ever since their development in the late 1980s, molecular switches based on the photoresponsive dithienylethene (DTE) architecture have attracted widespread attention as control elements in molecular devices and chemical systems [1]. This special interest over other classes of photoswitches is well deserved, and is due in part to the high fatigue resistance of the ring-closing and ring-opening photoreactions (Scheme 1.1), which reversibly generate two isomers. Also, the two isomers (‘ring-open’ and ‘ring-closed’) tend not to interconvert in the absence of light and, most importantly, possess markedly different optical and electronic properties. The most obvious change is in the colour of solutions, crystals and films containing DTE compounds [2]. However, numerous other useful differences in optical characteristics (emission [3] and optical rotation [4] of light), magnetism [5] and molecular and bulk conductivity [6] have been exploited in a remarkable number of derivatives to exert control over practical molecular systems. A few representative examples are listed in Table 1.1.
Scheme 1.1 The reversible, photochemical 6π electrocyclization reactions of the 1,3,5-hexatriene and 1,4-hexadiene isomers of the dithienylcyclopentene architecture.
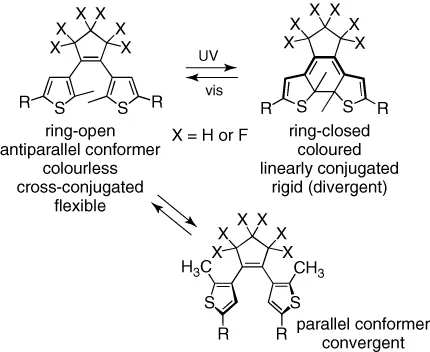
Table 1.1 Optical, Electronic and Bulk Properties Regulated by DTE Derivatives. All DTEs Exhibit an Intrinsic Modulation of their Absorption Characteristics, therefore, Specific Examples have not been Included.
| Structure | Comments | References |
| Systems that modulate fluorescence | ||
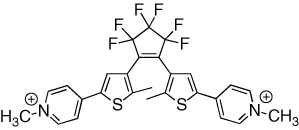 | Intrinsic fluorescence of the dye is reversibly modulated in a binary response | [7] |
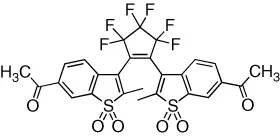 | Intrinsic fluorescence of the dye is reversibly modulated in a binary response with excellent fatigue resistance (>105 cycles) | [8] |
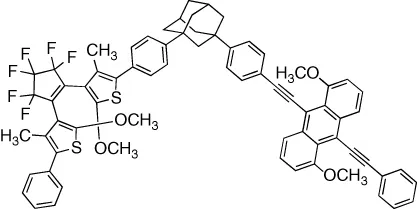 | Fluorescence of a pendant dye is reversibly modulated in a binary response | [9] |
| Control of single molecule emission in films is demonstrated | ||
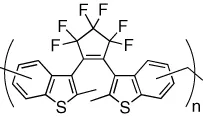 | Fluorescence of the polymeric DTE backbone is reversibly modulated in the solid state | [10] |
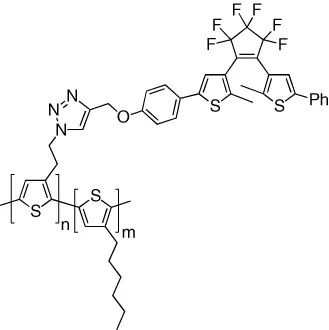 | Fluorescence of polythiophene is reversibly modulated by pendant DTEs | [11] |
| Amplified fluorescence quenching is demonstrated | ||
| Systems that modulate electron and hole transport | ||
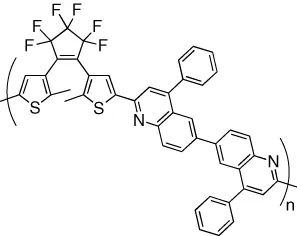 | Electron transport in the solid state is reversibly modulated with excellent performance | [12] |
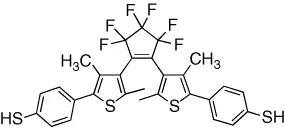 | Conductivity in a bulk nanoparticle network is reversibly modulated | [13] |
| The ring-closing or ring-opening reactions of certain DTEs can be suppressed by electronic interactions with metal surfaces. | ||
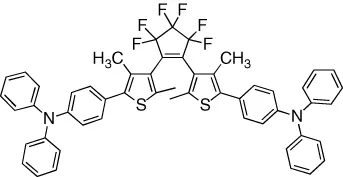 | Hole mobility and photocurrent in a bilayer device is reversibly modulated | [14] |
| Systems that modulate magnetism | ||
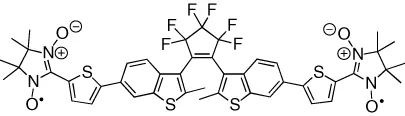 | Interactions between spin carriers on each end of the DTE backbone are reversibly modulated | [15] |
| Systems that modulate chirality | ||
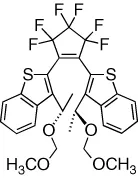 | Diastereoselective ring-closing is demonstrated | [16] |
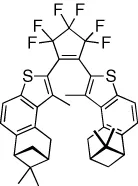 | Helical chirality is reversibly created through diastereoselective ring-closing | [17] |
| Systems that modulate bulk properties | ||
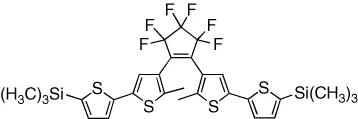 | Glass transition temperature of films is reversibly modulated | [18] |
| Selective metal deposition on crystalline areas is demonstrated | ||
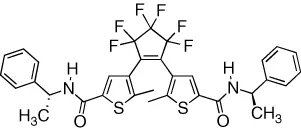 | Gelation of organic solvents by chiral helical fibres is reversibly modulated | [19] |
| The effect is amplified via aggregation | ||
Given the large number of reviews already in the literature that extensively cover examples of DTEs having the properties listed above [20–22], we decided to focus this chapter on two under-represented areas where the versatile photoresponsive DTE compounds can be used: electrochromism and controlling chemical/biochemical reactivity. In this chapter, we first highlight several examples of DTE derivatives that undergo ring-closing and/or ring-opening reactions when oxidized or reduced or irradiated with light (dual-mode photo-/electrochromic systems). The bulk of the chapter then concentrates on illustrating how the DTE backbone can be used to control chemical reactions (or often interactions) between molecules and how, in some cases, the opposite is also true: a chemical reaction or interaction can regulate the photochemistry of the DTE backbone. Several of the changes in molecular structure shown in Scheme 1.1 (such as flexibility, or proximity of pendant functional groups) are responsible for the success of DTEs in these last two areas.
1.2 Electrochemical Ring-Closing and Ring-Opening of DTEs
1.2.1 Electrochemical Behaviour of DTEs
Because the two thiophene heterocycles define many of the properties of the DTE backbone, the ring-open isomers tend to undergo irreversible oxidation at relatively high potentials (greater than 1 V) with accompanying electropolymerisation, as is typical for thiophene derivatives. Due to the creation of the linearly conjugated π-system upon photochemical cyclization, the ring-closed isomers typically undergo reversible oxidations at lower potentials (the absolute value depends on the derivative but are often 400–700 mV less positive), as expected for systems that have higher-energy highest-occupied molecular orbitals. Similarly, the reduction potentials for the ring-closed isomers are less negative than their ring-open counterparts (assuming the π-system created upon photoinduced cyclization is decorated with electron-accepting groups). These differences in redox potentials between the two DTE photoisomers can provide a possible mechanism for the observed selective quenching of fluorescence of pendant emissive dyes, however, only a few reports specifically ascribe electron transfer as the mechanism [23].
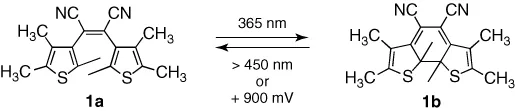
What is more pertinent to this chapter is the fact that some DTE derivatives undergo spontaneous ring-closing and ring-opening reactions when an appropriate voltage is applied and they are oxidized or reduced. The first example is compound 1b shown in Scheme 1.2 [24]. This compound undergoes the typical cyclization reaction when irradiated with UV light (1a → 1b). The reverse reaction is triggered either by exposing a solution of 1b to visible light or by applying a positive potential, which oxidizes the ring-closed isomer to its radical cation. Clearly, this species is unstable as it spontaneously ring opens to generate the radical cation of 1a, which undergoes electron transfer in a catalytic process that will be discussed in more detail.
Scheme 1.2 Ring-closing of DTE derivative 1a with UV light and ring-opening with visible light or electricity.
Other examples of DTE derivatives that show dual-mode photo- and electrochromism are listed in Table 1.2. In each case, how the DTE backbone is decorated with functional groups defines its dual-mode behaviour. For example, when the ‘outer positions’ (groups ‘A’ in Table 1.2) are aromatic rings (such as additional thiophenes) and the ‘inner positions’ (groups ‘B’ in Table 1.2) are alkyl groups, the ring-open isomers tend to cyclize when they are oxidized. Derivative 3 is an illustrative example and colourless solutions of it become coloured (blue) when a positive potential is applied to them (after the initially produced ring-closed radical cation is reduced) [25]. When the inner alkyl groups are replaced with aromatic rings, the opposite behaviour is observed and the ring-closed isomers un...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Preface
- List of Contributors
- Abbreviations
- Part I: Molecular Switching
- Part II: Switching in Containers, Polymers and Channels
- Part III: Molecular Switching in Logic Systems and Electronics
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Molecular Switches by Ben L. Feringa,Wesley R. Browne in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.