
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Selenium-based methods in synthetic chemistry have developed rapidly over the past years and are now offering highly useful tools for organic synthesis. Filling the gap for a comprehensive handbook and ready reference, this book covers all modern developments within
the field, including biochemical aspects. The chemistry chapters are organized according to the different reactivities of various selenium
compounds and reagents, with each chapter dealing with a special reaction type.
Also includes a table with 77Se NMR shifts to aid in practical problems.
From the Contents:
* Electrophilic and Nucleophilic Selenium
* Selenium Compounds in Radical Reactions
* Selenium-Stabilized Carbanions
* Selenium Compounds with Valency Higher than Two
* Selenocarbonyls
* Selenoxide Elimination and [2,3]-Sigmatropic Rearrangement
* Selenium Compounds as Ligands and Catalysts
* Biological and Biochemical Aspects of Selenium Compounds
the field, including biochemical aspects. The chemistry chapters are organized according to the different reactivities of various selenium
compounds and reagents, with each chapter dealing with a special reaction type.
Also includes a table with 77Se NMR shifts to aid in practical problems.
From the Contents:
* Electrophilic and Nucleophilic Selenium
* Selenium Compounds in Radical Reactions
* Selenium-Stabilized Carbanions
* Selenium Compounds with Valency Higher than Two
* Selenocarbonyls
* Selenoxide Elimination and [2,3]-Sigmatropic Rearrangement
* Selenium Compounds as Ligands and Catalysts
* Biological and Biochemical Aspects of Selenium Compounds
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Organoselenium Chemistry by Thomas Wirth in PDF and/or ePUB format, as well as other popular books in Ciencias físicas & Química física y teórica. We have over one million books available in our catalogue for you to explore.
Information
1
Electrophilic Selenium
1.1 General Introduction
During the last few decades, organoselenium compounds have emerged as important reagents and intermediates in organic synthesis.
Selenium can be introduced as an electrophile, as a nucleophile, or as a radical and generally it combines chemo-, regio-, and stereoselectivity with mild experimental conditions. Once incorporated, it can be directly converted into different functional groups or it can be employed for further manipulation of the molecule.
Since the discovery in the late 1950s that species of type RSeX add stereospecifically to simple alkenes [1], electrophilic organoselenium compounds provided the synthetic chemist with useful and powerful reagents and the selenofunctionalization of olefins represents an important method for the rapid introduction of vicinal functional groups, often with concomitant formation of rings and stereocenters (Scheme 1.1a and b).
Scheme 1.1 The reactivity of electrophilic selenium reagents.
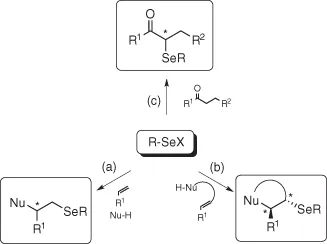
In addition, electrophilic selenium reagents can be also used for the α-selenenylation of carbonyl compounds (Scheme 1.1c) affording useful intermediates for the synthesis of α,β-unsaturated [2] derivatives or 1,2-diketones through a seleno-Pummerer reaction [3].
Oxidation of selenides to the corresponding selenoxide for the synthesis of α,β-unsaturated compounds represents a current topic in organic chemistry and has been used successfully also in structurally complex product synthesis. An example has been very recently reported in which the electrophilic selenenylation followed by an oxidative elimination represent a crucial step in the total synthesis of heptemerone G, a diterpenoid fungi-derived with interesting antibacterial activity (Scheme 1.2) [4].
Scheme 1.2 Electrophilic selenium reagent in the total synthesis of heptemerone G.
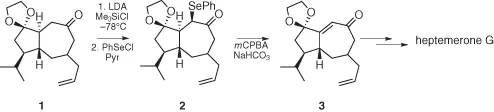
The kinetic lithium enolate 1, trapped as trimethysilyl derivatives, reacts with PhSeCl affording the selenide 2 that, after oxidation with metachloroperbenzoic acid, is converted into the enone 3 from which the heptemerone G can be prepared in some additional steps.
The treatment of selenides with tin hydrides, in the presence of AIBN, produces the homolytic cleavage of the carbon–selenium bond generating a carbon radical and opening the way for interesting radical reactions.
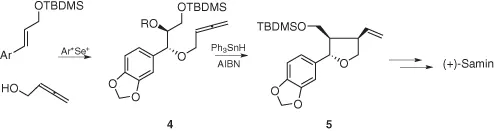
An elegant application was reported for the total synthesis of (+)-Samin (Scheme 1.3). The selenide 4 was subjected to radical deselenenylation conditions affording the tetrahydrofurane derivative 5 following a 5-exo-trig radical cyclization mechanism. From 5, (+)-Samin was obtained through a few classical steps [5].
Scheme 1.3 Electrophilic selenium reagent in the total synthesis of (+)-Samin.
The main aspects of organoselenium chemistry have been described in a series of books [6] and review articles and, in recent times, the synthesis of chiral selenium electrophiles as well as their applications in asymmetric synthesis represents a very interesting field of interests for many research groups [7].
In this chapter, we take in consideration some general aspects of the chemistry promoted by electrophilic selenium reagents by reporting selected examples and some more recent and innovative applications.
1.1.1 Synthesis of Electrophilic Selenium Reagents
Some phenylselenenyl derivatives such as chloride, bromide, and N-phenylselenophthalimide [8] are nowadays commercially available and represent the most common electrophilic reagents used to introduce selenium into organic molecules. Otherwise, in a more general procedure, very versatile precursors for the preparation of various electrophilic selenium species are the corresponding diselenides 6. They can be easily converted into selenenyl halides 7, 8 by treatment with sulfuryl chloride or chlorine in hexane and bromine in tetrahydrofuran, respectively (Scheme 1.4).
Scheme 1.4 Electrophilic selenium reagents.
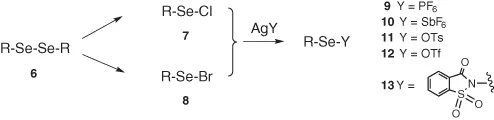
The use of halides in synthesis often gives rise to side processes due to the nucleophilicity of the halide anions. For this reason, a series of new selenenylating agents with nonhalide counterions have been reported.
Some of them were directly prepared starting from the appropriate selenenyl halide with silver salts such as hexafluorophosphate 9 [9], hexafluoroantimoniate 10 [10], tolylsulfonate 11 [11], and triflate 12 [12].
This latter is probably the most commonly used electrophilic selenium reagent even if, in many cases, the stoichiometric amount of trifluoromethanesulfonic acid formed is not compatible with the stability of the substrates and/or of the products. More recently, Tingoli reported a similar procedure to prepare the N-saccharin derivatives 13 containing a sulfonamide anion that is scarcely nucleophilic and generating saccharin that is a very weak acidic species [13].
In other cases, the electrophilic reagent can be more conveniently produced by the in situ oxidation of 6 with several inorganic reagents: KNO3 [14], CuSO4 [15], Ce(NH4)2(NO2)6 [16], Mn(OAc) [17], or nitrogen dioxide [18]. Among these, starting from diphenyl diselenide, (NH4)2S2O8 [19] produces the strongly electrophilic phenylselenenyl sulfate (PSS) 14 through a mechanism that reasonably involves an electron transfer or an SN2 reaction. A product derived from a single electron transfer has been proposed also as an intermediate in the reaction of diphenyl diselenide with 1,2-dicyanonaphthalene [20] that leads to the formation of the phenylselenenyl cation 15 as depicted in Scheme 1.5.
Scheme 1.5 Electrophilic selenium reagents through a single electron transfer mechanism.
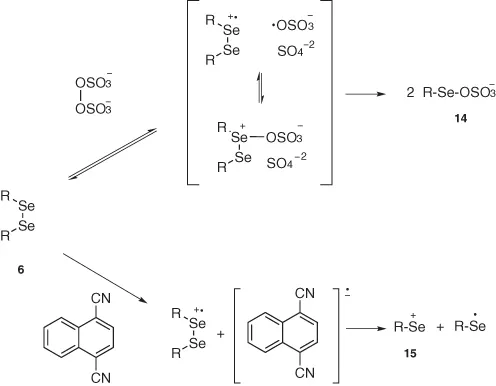
Some other organic oxidizing agents such as m-nitrobenzenesulfonyl peroxide [21], (bis[trifluoroacetoxy] iodo)benzene [22], (diacethoxy iodo)benzene [23], and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) [24] have been also used in some cases. The choice of the best reagent strongly depends on the chemical susceptibility of the substrate and its functional groups and is mainly dictated by the requirements of the addition reaction to be carried out.
In recent years, polymer-supported reagents have also attracted interest because they can provide attractive and practical methods for combinatorial chemistry and solid-phase synthesis.
Polymer-supported selenium reagents represent an interesting improvement for synthetic organic chemists because of their facile handling without the formation of toxic and odorous by-products. Some electrophilic selenium-based approaches for solid-phase chemistry have been reported by different groups and the use of these reagents allows easy purification and recycling of the reagent for a next reaction. In addition, it represents useful strategies especially for constructing libraries of heterocyclic derivatives [25]. Wirth and coworkers compared the efficiency of polystyrene, TentaGel, and mesopouros silica as a solid support for enantioselective electrophilic addition reactions [26].
In a recent application, polystyrene-supported selenenylbromide was reacted with methyl acrylate and a primary amine to afford in a one-pot procedure a resin that has been used to prepare libraries of 2-pyridones, 1,4-diazepines, 1,4 oxazepines [27], and other nitrogen heterocycles [28].
Even if the mild reaction conditions usually required for the selenenylation of unsatured substrates represent an attractive aspect for this chemistry, some of these conver...
Table of contents
- Cover
- Related Titles
- Title page
- Copyright page
- Preface
- List of Contributors
- 1 Electrophilic Selenium
- 2 Nucleophilic Selenium
- 3 Selenium Compounds in Radical Reactions
- 4 Selenium-Stabilized Carbanions
- 5 Selenium Compounds with Valency Higher than Two
- 6 Selenocarbonyls
- 7 Selenoxide Elimination and [2,3]-Sigmatropic Rearrangement
- 8 Selenium Compounds as Ligands and Catalysts
- 9 Biological and Biochemical Aspects of Selenium Compounds
- 77Se NMR Values
- Index