
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Principles and Applications of Lithium Secondary Batteries
About this book
Lithium secondary batteries have been key to mobile electronics since 1990. Large-format batteries typically for electric vehicles and energy
storage systems are attracting much attention due to current energy and environmental issues. Lithium batteries are expected to play a central
role in boosting green technologies. Therefore, a large number of scientists and engineers are carrying out research and development on
lithium secondary batteries.
The book is written in a straightforward fashion suitable for undergraduate and graduate students, as well as scientists, and engineers
starting out in the field. The chapters in this book have been thoroughly edited by a collective of experts to achieve a cohesive book with a consistent style, level, and philosophy. They cover a wide range of topics, including principles and technologies of key materials such as the
cathode, anode, electrolyte, and separator. Battery technologies such as design, manufacturing processes, and evaluation methods as well as applications are addressed. In addition, analytical methods for determining electrochemical and other properties of batteries are also included.
Hence, this book is a must-have for everyone interested in obtaining all the basic information on lithium secondary batteries.
storage systems are attracting much attention due to current energy and environmental issues. Lithium batteries are expected to play a central
role in boosting green technologies. Therefore, a large number of scientists and engineers are carrying out research and development on
lithium secondary batteries.
The book is written in a straightforward fashion suitable for undergraduate and graduate students, as well as scientists, and engineers
starting out in the field. The chapters in this book have been thoroughly edited by a collective of experts to achieve a cohesive book with a consistent style, level, and philosophy. They cover a wide range of topics, including principles and technologies of key materials such as the
cathode, anode, electrolyte, and separator. Battery technologies such as design, manufacturing processes, and evaluation methods as well as applications are addressed. In addition, analytical methods for determining electrochemical and other properties of batteries are also included.
Hence, this book is a must-have for everyone interested in obtaining all the basic information on lithium secondary batteries.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Principles and Applications of Lithium Secondary Batteries by Jung-Ki Park in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Electrical Engineering & Telecommunications. We have over one million books available in our catalogue for you to explore.
Information
Edition
1Chapter 1
Introduction
With the proliferation of mobile telecommunication devices arising from remarkable developments in information technology (IT), the twenty-first century is moving toward a ubiquitous society, where high-quality information services are available regardless of time and place. The establishment of a ubiquitous society can be traced back to lithium secondary batteries, which were first commercialized in the early 1990s. Compared to other secondary batteries, lithium secondary batteries not only have higher working voltage and energy density, but also have long service life. Such superior characteristics enable secondary lithium batteries to fulfill complex requirements for diversified growth in devices. Global efforts are underway to further develop the existing technology of lithium secondary batteries and expand their use from eco-friendly transportation to various fields, such as power storage, health care, and defense.
A fundamental and systematic understanding of lithium secondary batteries is essential for the continuous development of related technologies along with technological innovation.
1.1 History of Batteries
A battery can be defined as a system that uses electrochemical reaction to directly convert the chemical energy of an electrode material into electric energy. The battery was first described in an 1800 study by Volta, an Italian professor at the University of Pavia, and published by the Royal Society of London. In 1786, Galvani of Italy discovered that touching a frog's leg with a metal object caused muscular convulsions. He claimed that “animal electricity” was generated from within the frog and transported through its muscles. Volta, who doubted the credibility of animal electricity, confirmed that the animal's body fluid merely served as an electrolyte between two different metals. In 1800, Volta invented the voltaic pile, in which an electric current is produced by connecting the two ends of a stack of two metal disks separated by cloth soaked in an alkaline solution. This was the first form of the battery as we know it today [1].
A 2000-year-old clay jar, believed to be the earliest specimen of a battery, was discovered at a historic site near Baghdad in 1932 (Figure 1.1b). This clay jar had a height of 15 cm and contained a copper cylinder that was held in place by copper and iron rods. The rods had been corroded by acid. Although the artifact is accepted by some scholars as a primitive cell, it is uncertain whether it was indeed used for such a purpose.
Figure 1.1 The voltaic pile (a) and the Baghdad battery (b).
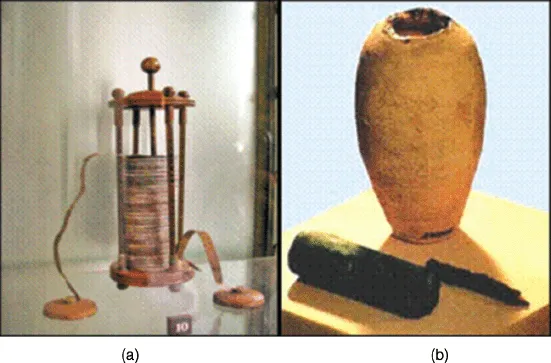
Batteries can be classified into primary batteries, which are used once and disposed, and secondary or rechargeable batteries, which can be recharged and used multiple times. Since the invention of the voltaic pile, various batteries have been developed and commercialized.
The first widely used primary battery was the Leclanché (or manganese) cell invented in 1865 by Leclanché, a French engineer. The Leclanché cell, containing a zinc anode, a manganese dioxide (MnO2) cathode, and an acidic aqueous electrolyte of ammonium chloride (NH4Cl) and zinc chloride (ZnCl2), had a wide range of applications with an electromotive force of 1.5 V. Later, the aqueous electrolyte in the Leclanché cell was replaced with an alkaline electrolyte of potassium hydroxide (KOH). This became the alkaline battery, which enhanced capacity and discharge, with the same voltage. New types of primary batteries later emerged, such as zinc–air batteries (1.4 V) and silver oxide batteries (1.5 V). The performance of primary batteries was greatly improved in the 1970s when 3 V lithium primary batteries with lithium as an anode became commercialized.
The oldest type of secondary batteries is lead–acid battery, invented by French physicist Planté in 1859. Lead–acid batteries have a lead peroxide anode, a lead cathode, and weak sulfuric acid as an electrolyte. With an electromotive force of 2 V per cell, they are commonly used as storage batteries in motor vehicles. When NiCd (1.2 V) batteries became widespread in 1984, they began to replace primary batteries in small electric appliances [2]. However, owing to the harmful environmental effects of cadmium, NiCd batteries are not as widely used today.
In the early 1990s, NiMH (1.2 V) cells were favored over NiCd batteries for their eco-friendliness and enhanced performance. This was followed by the emergence of 3 V lithium secondary batteries with greatly improved energy density. Compact and lightweight lithium secondary batteries soon dominated the market for portable devices, including cell phones, laptops, and camcorders [3].
1.2 Development of Cell Technology
After the invention of the voltaic pile in 1800, two significant milestones were reached in the 200-year history of cell technology. One was the development of primary batteries into secondary batteries and the other was the advancement to a working voltage of 3 V. Lithium secondary batteries, which use lithium ions as the main charge carrier, can maintain a high average discharge voltage of 3.7 V despite being lightweight. With the highest energy density among all currently available batteries, lithium secondary batteries have led the revolution of cell technology.
Looking at the changes in energy density with developments in secondary cell technology, lead–acid batteries have a specific energy of 30 Wh/kg and energy density of 100 Wh/l, whereas the energy density of lithium secondary batteries has shown an annual increase of 10%. At present, cylindrical lithium secondary batteries have a specific energy of 200 Wh/kg and energy density of 600 Wh/l (Figure 1.2). The specific energy of lithium secondary batteries is five times that of lead–acid batteries and three times higher than that of NiMH cells [4].
Figure 1.2 Changes in energy density with developments in cell technology.
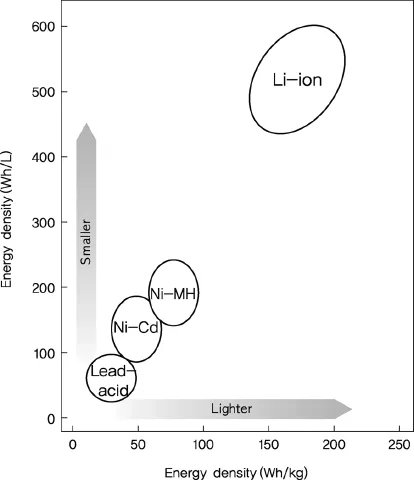
NiMH cells, a type of secondary batteries, have limited working voltage and energy density, but are attractive in terms of application to hybrid electric vehicles (HEVs) owing to their high stability. Recently, the advent of plug-in hybrid electric vehicles (PHEVs) and electric vehicles (EVs) has brought greater attention to lithium secondary batteries, which have higher energy and output compared to NiMH cells. Because secondary batteries in electric vehicles should offer fast charging time, lightweight, and high performance, future technology developments surrounding lithium secondary batteries are likely to be highly competitive. We can expect revolutionary and continuous technological progress that will overcome current limitations.
1.3 Overview of Lithium Secondary Batteries
For a cell to be characterized as a secondary battery, the anode and cathode have to repeat charging and discharging. The electrode structure should be kept stable during the insertion and extraction of ions within electrodes, while an electrolyte acts as an ion transfer medium. The charging of a lithium secondary battery is illustrated in Figure 1.3.
Figure 1.3 Movement of Li+ in an electrolyte and insertion/extraction of Li+ within electrodes in a lithium secondary battery.
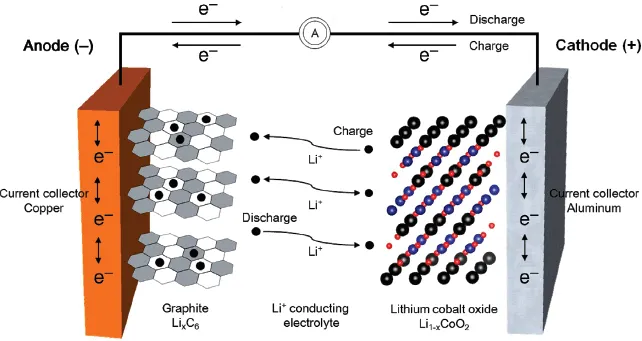
Charge neutrality occurs when ions flowing into electrodes collide with electrons entering through a conductor, thus forming a medium to store electric energy in the electrodes. Furthermore, the rate of reactions is increased as ions from the electrolyte are drawn to the electrodes. In other words, the overall reaction time of a cell heavily depends on the movement of ions between electrolyte and electrodes. The amount of ions inserted into electrodes for charge neutrality determines the electrical storage capacity. Ultimately, the types of ions and materials are main factors that influence the amount of electric energy to be stored. Cells based on lithium ions (Li+) are known as lithium secondary batteries.
Lithium, which is the lightest of all metals and has the lowest standard reduction potential, is able to generate a working voltage greater than 3 V. With a high specific energy and energy density, it is suitable for use as an anode material. Since the working voltage of lithium secondary batteries is greater than the decomposition voltage for water, organic electrolytes should be used instead of aqueous solutions. Materials that facilitate the insertion and extraction of Li+ ions are appropriate as electrodes.
Lithium secondary batteries use a transition metal oxide as an cathode and carbon as a anode. The electrolyte of lithium ion batteries (LIBs) is held in an organic solvent, while that of lithium ion polymer batteries (LIPBs) is a solid polymer composite.
As shown in Figure 1.4, commercialized lithium secondary batteries can be classified according to cell shape and component materials. The various forms of batteries include cylindrical laptop batteries, prismatic cells for portable devices, single-cell coin-shaped batteries, and pouch-shaped cells cased in aluminum plastic composites [4].
Figure 1.4 Different shapes of lithium secondary batteries: (a) cylindrical, (b) coin, (c) prismatic, (d) pouch [4]. Reprinted by Permission from Macmillan Publishers Ltd: [4], copyright 2001.
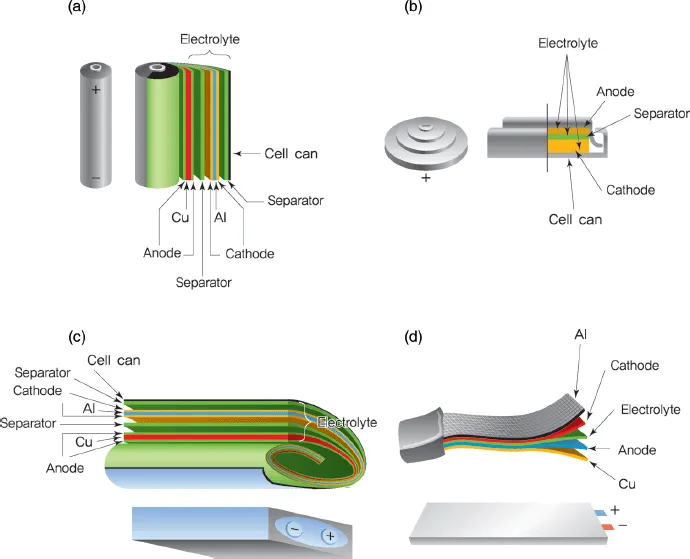
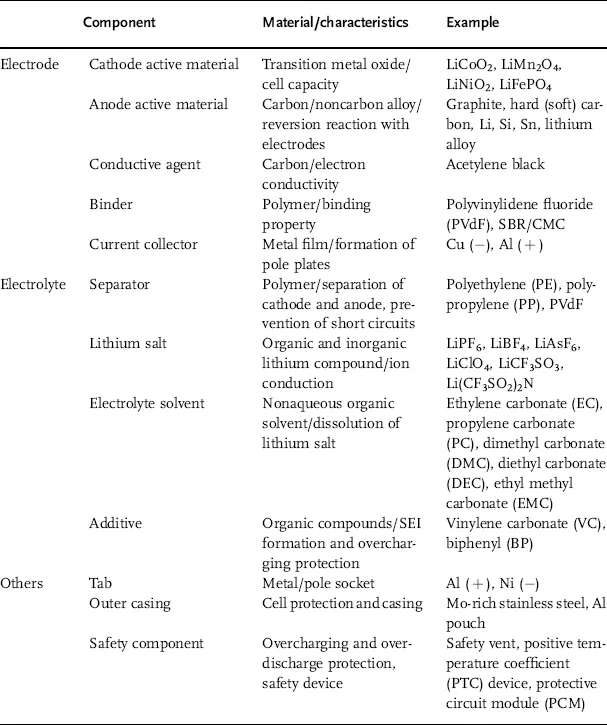
Table 1.1 shows the key components of a lithium secondary battery. Its materials can be described as follows. Because lithium is removed from the lattice structure and released as ions, stable transition metal oxides are used as cathodes. Anode materials should have a standard reduction potential similar to that of lithium so as to stabilize the released ions and provide a large electromotive force. The electrolyte consists of a lithium salt in an organic solvent, thus maintaining electrochemical and thermal stability within the range of the working voltage. In addition, separators made of polymers or ceramics have a high-temperature melt integrity, which prevents short circuits caused by electrical contact between the cathode and the anode.
Table 1.1 Characteristics and examples of key components in a lithium secondary battery.
1.4 Future of Lithium Secondary Batteries
To date, the development of lithium secondary batteries has focused on small electric appliances and portable IT devices. Lithium secondary batteries are expected to build upon these achievements and create new applications described by buzzwords, such as “green energy,” “wireless c...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- List of Contributors
- Preface
- Chapter 1: Introduction
- Chapter 2: The Basic of Battery Chemistry
- Chapter 3: Materials for Lithium Secondary Batteries
- 3.1 Cathode Materials
- 3.2 Anode Materials
- 3.3 Electrolytes
- 3.4 Interfacial Reactions and Characteristics
- Chapter 4: Electrochemical and Material Property Analysis
- 4.1 Electrochemical Analysis
- 4.2 Material Property Analysis
- Chapter 5: Battery Design and Manufacturing
- Chapter 6: Battery Performance Evaluation
- Index