
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Clinical Pediatric Endocrinology
About this book
This revised edition of Charles Brook's Handbook of Clinical Pediatric Endocrinology provides endocrinologists and pediatricians in training with a fully up-to-date clinical guide presenting evidence-based practice in the diagnosis, treatment, and management of pediatric endocrine disorders.New chapters include "Endocrine complications of chronic disease" and "Endocrine neoplasia." In addition, the chapter structure has been revamped for easier accessand now includes: a key points overview, multiple-choice questions for self-assessment, common errors/pitffalls (in treatment, diagnosis, etc.) boxes, a key weblinks box, a table comparing different society guidelines, diagnostic decision trees, therapeutics decision trees, and a summary.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Handbook of Clinical Pediatric Endocrinology by Charles G. D. Brook,Mehul Dattani in PDF and/or ePUB format, as well as other popular books in Medicine & Endocrinology & Metabolism. We have over one million books available in our catalogue for you to explore.
Information

CHAPTER 1
The Relevance of Molecular Biology to Clinical Practice
Key learning points
1. The inheritance of genetic disorders in humans may be mendelian or more complex.
2. The human genome contains around 30,000–40,000 genes, and its complexity is further increased by the use of alternative promoters, epigenetic phenomena and alternative splicing.
3. Further complexity is imparted by disorders of imprinting, mitochondrial disorders, mosaicism, digenic/oligogenic inheritance, sex-influenced phenotypes and variability of penetrance and expressivity.
4. Novel genetic techniques such as array-CGH and next-generation sequencing will revolutionize molecular diagnostics over the next few years.
5. Molecular diagnosis is increasingly important for optimal medical management of a number of human disorders.
An understanding of and a facility with the molecular basis of many endocrine and non-endocrine disorders is essential to the practice of pediatric endocrinology in the 21st century and knowledge is accumulating rapidly (see Online Mendelian Inheritance in Man [OMIM], a comprehensive catalog of human genes and genetic disorders in order to keep pace with this field: www.ncbi.nlm.nih.gov/sites/entrez?db=omim) (see also Web links box). With the exception of simple trauma, every disease has a genetic component.
In monogenic disorders, for example, congenital adrenal hyperplasia (CAH), the genetic component is the major etiological factor. Multiple genes operating in conjunction with environmental and lifestyle factors contribute to the pathogenesis of polygenic or multifactorial disorders. Genetic factors also influence the manifestation of disease directly through the genetic defect or indirectly by defining the host’s susceptibility and resistance to an environmental disease such as infection.
Genetics (Fig. 1.1) is the science of heredity and variation and has heretofore focused on chromosomal abnormalities and inborn errors of metabolism. Analysis of the transmission of human traits and disease within families has led to understanding many monogenic disorders. Diabetes mellitus type 2, obesity, hypertension, heart disease, asthma and mental illnesses are complex and the genetic susceptibilities to these disorders are influenced by exogenous factors interacting with genetic susceptibilities.
Figure 1.1 Overview of genomics, transcriptomics, proteomics, metabolomics, bioinformatics and systems biology.
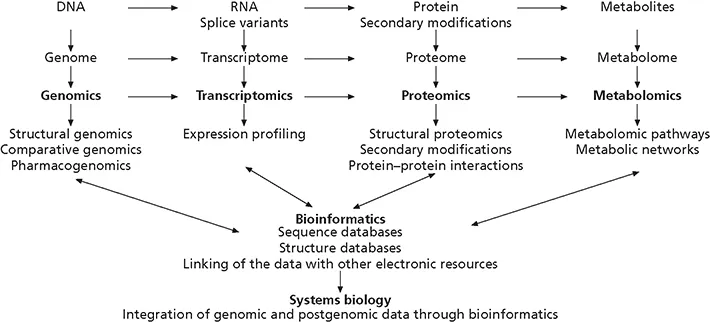
Phenotype can also be influenced by genetic and environmental modifiers in monogenic disorders. For example, the expression of the phenotype in monogenic forms of diabetes mellitus due to mutations in the maturity onset of diabetes in the young (MODY) genes is influenced by factors such as diet and physical activity.
The term genome, introduced before the recognition that DNA is the genetic material, designates the totality of all genes on all chromosomes in the nucleus of a cell. Genomics refers to the discipline of mapping, sequencing and analyzing genomes. Genome analysis can be divided into structural and functional genomics. The analysis of differences among genomes of individuals of a given species is the focus of comparative genomics. The complement of messenger RNAs (mRNAs) transcribed by the cellular genome is called the transcriptome and the generation of mRNA expression profiles is referred to as transcriptomics. Epigenetic alterations and chemical modifications of DNA or chromatin proteins influence gene transcription. The sum of all epigenetic information defines the epigenome, which, in contrast to the genome, is highly variable between cells and changes within a single cell over time. Epigenetic modifications lead to phenotypic changes without alteration of DNA sequence, and may be heritable.
The term proteome describes all the proteins expressed and modified following expression by the entire genome in the lifetime of a cell. Proteomics refers to the study of the proteome using techniques of large-scale protein separation and identification. The field of metabolomics aims at determining the composition and alterations of the metabolome, the complement of low molecular weight molecules. The relevance of these analyses lies in the fact that proteins and metabolites function in modular networks rather than linear pathways. Hence, any physiological or pathological alteration may have many effects on the proteome and metabolome.
Pharmacogenomics, which involves the analysis of the genetic factors determining the response of an individual to a particular therapeutic agent, is emerging as a major new field. Genetic polymorphisms can not only influence the effects of medications but also result in variable absorption, distribution, metabolism, and excretion of a drug.
The growth of biological information has required computerized databases to store, organize, annotate and index the data which has led to the development of bioinformatics, the application of informatics to (molecular) biology. Computational and mathematical tools are essential for the management of nucleotide and protein sequences, the prediction and modeling of secondary and tertiary structures, the analysis of gene and protein expression and the modeling of molecular pathways, interactions and networks.
The integration of data generated by transcriptomic, proteomic, epigenomic and metabolomic analyses through informatics is an emerging discipline aimed at understanding phenotypic variations and creating comprehensive models of cellular organization and function. These efforts are based on the expectation that an understanding of the complex and dynamic changes in a biological system may provide insights into pathogenic processes and the development of novel therapeutic strategies and compounds.
The human genome
Genes were identified because they conferred specific traits transmitted from one generation to the next. They are functional units regulated by transcription and encode messenger (m)RNA which is subsequently translated into protein. Biological roles have been described for other RNAs, such as transfer (t)RNA, ribosomal (r)RNA and micro (mi)RNA. The latter are small non-coding RNAs that regulate gene expression by targeting mRNAs of protein coding genes or non-coding RNA transcripts. Micro RNAs also have an important role in developmental and physiological processes and can act as tumour suppressors or oncogenes in the ontogenesis of cancers.
The Human Genome Project led to the complete publication of the human genome DNA sequence in 2003, and revealed that 30,000–40,000 genes account for 10–15% of genomic DNA. Much of the remaining DNA consists of highly repetitive sequences, the function of which remains incompletely understood. Genes are unevenly distributed along the various chromosomes, and range in size from a few hundred base pairs to more than 2 million base pairs. The number of genes identified was surprisingly small, which suggests that the use of various promoters, alternative splicing of genes, and epigenetic phenomena account for the rich phenotypic diversity observed in humans.
Human genetics aims at understanding the role of common genetic variants in susceptibility to common disorders by identifying, cataloging and characterizing gene variants which include short repetitive sequences in regulatory or coding regions and single nucleotide polymorphisms (SNPs), changes in which a single base in the DNA differs from the usual base at that position. SNPs occur roughly every 300 base pairs and most are found outside coding regions.
Single nucleotide polymorphisms within a coding sequence can be synonymous (i.e. not altering the amino acid code) or non-synonymous. There are roughly 3 million differences between the DNA sequences of any two copies of the human genome. SNPs that are in close proximity are inherited together as blocks referred to as haplotypes, and this forms the basis of the International HapMap project. The identification of approximately 10 million SNPs that occur commonly in the human genome through the International HapMap Project is of great relevance for genome-wide association studies.
Structure and function of genes
The structure of a typical gene consists of regulatory regions followed by exons and introns and downstream untranslated regions (Fig. 1.2). Regulatory sequences controlling gene expression characteristically lie upstream (5’) of the transcription start site, although a number of regulatory regions have now been identified in the introns or downstream (3’) of the coding region of the gene.
Figure 1.2 Schematic representation of a gene and of the events leading to the synthesis of a peptide hormone.
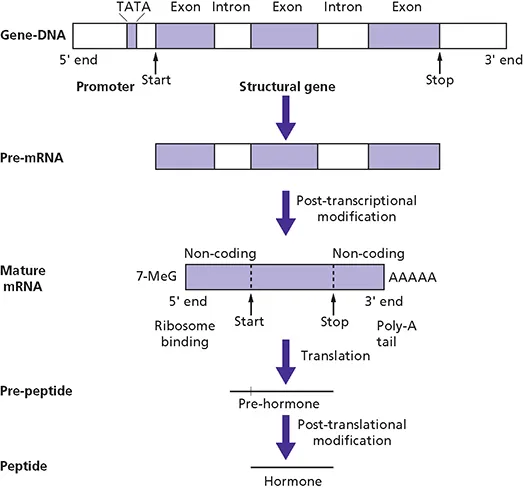
The regulatory DNA sequences of a gene upstream of the coding region are referred to as the promoter region, which contains specific sequences called response elements that bind transcription factors. Some are ubiquitous. Others are cell-specific. Gene expression is controlled by additional regulatory elements, such as enhancers and locus control regions which may be located far away from the promoter region. The transcription factors that bind to the promoter and enhancer sequences provide a code for regulating transcription that is dependent on developmental state, cell type and endogenous and exogenous stimuli. Transcription factors interact with other nuclear proteins and generate large regulatory complexes that ultimately activate or repress transcription.
Transcription factors account for about 30% of all expressed genes. Mutations in them cause a number of endocrine and non-endocrine genetic disorders. Because a given set of transcription factors may be expressed in various tissues, it is not uncommon to observe a syndromic phenotype. The mechanism by which transcription factor defects cause disease often involves haploinsufficiency, a situation in which a single copy of the normal gene is incapable of providing sufficient protein production to assure normal function. Biallelic mutations in such a gene may result in a more pronounced phenotype. For example, monoallelic mutations in the transcription factor HESX1 result in various constellations of pituitary hormone deficiencies and the phenotype is variably penetrant among family members with the same mutation. Inactivating mutations of both alleles of HESX1 cause familial septo-optic dysplasia and/or combined pituitary hormone deficiency (de Morsier syndrome).
Gene expression can be influenced by epigenetic events, such as X-inactivation and imprinting, i.e. a marking of genes that results in monoallelic expression depending on their parental origin. In this situation, DNA methylation leads to silencing, i.e. suppression of gene expression on one of the chromosomes. Genomic imprinting has an important role in the pathogenesis of several genetic disorders such as Prader–Willi or Silver–Russell syndromes and Albright hereditary osteodystrophy.
Molecular biology in diagnosis
Analysis of chromosomes, DNA and RNA
Analyses of large alterations in the genome are possible using cytogenetics, fluorescence in situ hybridization (FISH), Southern blotting, high-throughput genotyping and sequencing. More discrete sequence alterations rely heavily on the use of the polymerase chain reaction (PCR), which permits rapid genetic testing and mutational analysis with small amounts of DNA extracted from solid tissues, nucleated blood cells, leukocytes, buccal cells or hair roots. Reverse transcription PCR (RT-PCR) transcribes RNA into a complementary DNA strand, which can then be amplified by PCR. RT-PCR can be used for sequence analyses of the coding regions and to detect absent or reduced levels of mRNA expression resulting from a mutated allele.
Screening for point mutations can be performed by numerous methods, such as sequencing of DNA fragments amplified by PCR, recognition of mismatches between nucleic acid duplexes or electrophoretic separation of single- or double-stranded DNA. Most traditional diagnostic methods focus on single genes. Novel techniques for the analysis of mutations, genetic mapping and mRNA expression profiles are evolving. Chip techniques allow hybridization of DNA or RNA to hundreds of thousands of probes simultaneously. Microarrays are being used for mutational analysis of human disease genes, for the identification of viral seq...
Table of contents
- Cover
- Title page
- Copyright page
- Preface
- List of Abbreviations
- CHAPTER 1: The Relevance of Molecular Biology to Clinical Practice
- CHAPTER 2: Hormones: their Nature, Action and Measurement
- CHAPTER 3: The Hypothalamo-Pituitary Axis
- CHAPTER 4: Endocrine Problems of Infancy
- CHAPTER 5: The Management of Growth Disorders
- CHAPTER 6: The Management of Puberty Disorders
- CHAPTER 7: The Thyroid Gland
- CHAPTER 8: The Adrenal Gland
- CHAPTER 9: Disorders of Calcium and Bone Metabolism
- CHAPTER 10: Water Balance
- CHAPTER 11: Hypoglycemia
- CHAPTER 12: Obesity and Type 2 Diabetes Mellitus
- CHAPTER 13: Type 1 Diabetes Mellitus
- CHAPTER 14: Endocrine Neoplasia
- CHAPTER 15: Tests and Normal Values in Pediatric Endocrinology
- Appendix 1: Syndrome-Specific Growth Charts
- Appendix 2: Normal Values
- Index