
eBook - ePub
The Dose Makes the Poison
A Plain-Language Guide to Toxicology
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This new edition of a widely-read and highly-acclaimed book broadens the scope of its predecessors from a heavy focus on industrial chemicals as toxicants to include drugs, food additives, cosmetics and other types of compounds that people are exposed to daily. Also new to the 3rd edition are newer issues-of-the-day such as nanoparticulate toxicants, second hand smoke, food contamination, lead in toys, and others. As such, the book provides the basics of toxicology in easy-to-understand language as well as a fuller understanding of the daily insults to which our bodies are subjected.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access The Dose Makes the Poison by Patricia Frank,M. Alice Ottoboni in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Public Health, Administration & Care. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
WHAT ARE CHEMICALS?
The word chemical has become a dirty word in our modern American vocabulary. Our public media provide us daily with advice or warnings about the presence of chemicals in our food, air, and water and the harm they are doing to us and the world we live in. As a result, the word chemical conjures up visions of damage, debility, disease, and death in the minds of many people. In order to understand the threats posed by chemicals—a prerequisite to wisely protecting ourselves and our environment from their adverse effects—we must clarify or reform our concept of chemical.
ATOMS AND MOLECULES
All matter is composed of chemical elements. An individual unit of an element is called an atom. Atoms are the basic building blocks for all substances. Approximately 90 different kinds of stable elements are found in nature. Examples of elements are hydrogen, oxygen, carbon, nitrogen, gold, and silver. A complete listing of all of the elements, including those that are unstable (radioactive), can be found in any good dictionary. The periodic table gives detailed information about all the elements and the relationships among them. A multicolored diagram of the periodic table and an explanation of how this table is constructed can be found at the Los Alamos National Laboratory Web site (http://periodic.lanl.gov) or the University of Sheffield Web site (www.webelements.com). Appendix A describes the concept of Avogadro’s number and molecular weights for those who might be interested.
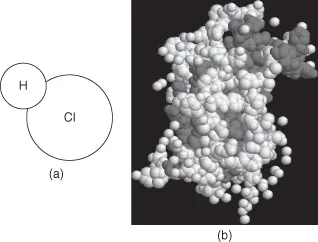
When two or more atoms (usually of different elements) are linked together by chemical bonding, they form units called molecules. A substance composed of molecules all of the same kind is called a compound. Water, salt, and sugar are examples of compounds. The number of different kinds of molecules that can be formed by the combination of from two to many thousands of atoms, from more than 90 different elements, is astronomical. Figure 1-1 shows the structures of a very simple and a very complex molecule. All substances are composed of chemical and physical combinations of atoms (elements) and molecules (compounds). Thus, everything in our physical world is chemical—the food we eat, the water we drink, the clothes we wear, the medicines we take, the cosmetics we use, the plants in our garden, our furniture, our homes, our automobiles, and even ourselves. Our entire physical world is composed of chemicals.
FIGURE 1-1 (a) Hydrochloric acid, a simple compound; (b) growth hormone, a complex compound.
[Part (b) from Wikimedia open source, http://commons.wikimedia.org.]
NATURAL CHEMICALS
The total number of chemical compounds in our universe that occur naturally will never be known exactly, but, from the millions that have been identified thus far, we know that the total number is huge. Natural chemicals may be organic (i.e., containing carbon) or inorganic. Our inanimate world is an inorganic world. It is composed of a great number of mineral substances in which all of the elements, except for a few radioactive elements that have been created by nuclear scientists, are represented.
Our living world is composed primarily of organic compounds, the diversity of which is tremendously greater than that in our inorganic world. The number of natural organic compounds that has been identified thus far, although very large, is probably negligible compared to the number of those yet unidentified. Many of these as-yet-unidentified organic chemicals—components of the trees, shrubs, and other plants of the rain forests—could well be of great value to medical and pharmaceutical sciences.
One small segment of our organic world, food plants and animals, provide us with the nutrients that we use to build and repair our bodies. However, the plants and animals we use for food contain many more natural chemicals than just the nutrients we require. Since it is impossible to separate nutrients from non-nutrients in our foods, we depend on our bodies to do this work for us. There are many kinds and quantities of nonnutrients in our foods, particularly our plant foods. The animals we use for food have already done the job for us of selecting nutrients and eliminating most of the nonnutrients from plants.
Among the natural chemicals that we eat, many can cause adverse effects if consumed in excess. In fact, there is probably no food that does not contain some potentially harmful natural chemical. This fact is the basis for an annual project of the American Council on Science and Health (ACSH).* Every fall the ACSH publishes a typical Thanksgiving menu accompanied by identification of the naturally occurring toxic or carcinogenic chemicals present in each food on it (found at www.acsh.org). For example, taken from the 2009 menu are heterocyclic amines, acrylamide, benzo(a)pyrene, ethyl carbamate, dihydrazines, d-limonene, safrole, and quercetin glycosides—and this just from the turkey with stuffing! If you are keeping to a vegetarian diet, then the 2009 menu shows salad may contain aniline, caffeic acid, benzaldehyde, hydrogen peroxide, quercetin glycosides, and psoralens.
An interesting method for ranking the potential health effects from exposure to such toxicants that occur naturally in foods was developed by Bruce Ames and his colleagues at the University of California, Berkeley. Dr. Ames has written numerous articles for both scientific and popular publications reviewing the subject of naturally occurring toxicants and their carcinogenic hazards. Rankings are based on data from the scientific literature as well as from Dr. Ames’s own laboratory, using accepted methods of risk assessment. These rankings are one approach to the evaluation of relative health risks posed by suspected carcinogens, both natural and synthetic.
SYNTHETIC CHEMICALS
Humans, in their ingenuity, have been able to take the basic building blocks of which all matter is composed and link them together in new combinations to produce compounds not found in nature. Thus, we have a host of synthetic substances, primarily organic, available to us, which we put to a seemingly endless variety of uses—pharmaceuticals, pesticides, and polymers of all sorts, including the common household plastics with which we are so familiar.
The term organic has been extensively used by the health-food industry to mean one thing and used by chemists to mean another; as a result, the term is generally misunderstood by the public. Organic has come to mean something (usually food) that is naturally occurring or produced without the use of pesticides or other synthetic chemicals, such as hormones. Scientifically, organic chemicals are simply chemicals composed primarily of the element carbon, independent of whether they are natural or synthetic. It comes as a shock to many people that almost all synthetic chemicals, including pesticides, are organic chemicals. The term organic was coined long before the birth of modern chemistry.
Early scientists who studied the composition of matter recognized that substances produced by living organisms were different from all other chemicals then known to humans. They called the former organic (derived from organisms) as opposed to the latter, which they classified as inorganic. Early in the nineteenth century, scientists discovered that the element carbon was present in all organic compounds; hence carbon chemistry became synonymous with organic chemistry.
The great complexity of carbon chemistry, relative to inorganic chemistry, the large size and complicated structures of many organic compounds, their great number and variety, combined with the fact that organic chemicals were found only in living organisms or products of living organisms led the early-day chemists to endow organic chemicals with mystical properties. They considered that the laws that governed the behavior of inorganic chemicals did not apply to organic chemicals; humans could synthesize—that is, manufacture—compounds such as nitrous oxide and hydrochloric acid but were incapable of synthesizing organic compounds in the laboratory at that time.
The special properties of organic chemicals were attributed to the action of a supernatural force, the “vital force,” as distinct from the crude and vulgar forces that governed inorganic chemicals. Jöns Berzelius, a noted chemist of the early nineteenth century, wrote that the vital force was unrelated to inorganic elements and determined none of their characteristic properties. Berzelius considered that the vital force was a mysterious property beyond comprehension.
The birth of synthetic organic chemistry occurred at about the time of Berzelius’s writing, with the first laboratory synthesis of an organic chemical, using basic chemicals as starting materials. The first synthetic organic chemical was oxalic acid, made by the German chemist Friedrich Wohler. A short time later in 1824, Wohler also synthesized urea. After this accomplishment, Wohler wrote to Berzelius to tell him that he had prepared urea, a chemical found in the urine of animals, “without requiring a kidney or animal, either man or dog.”
The notion that organic and inorganic chemicals were qualitatively different persisted for decades after the revolutionary demonstration that humans could, indeed, synthesize organic chemicals. The science of chemistry was greatly retarded until the chemical properties of carbon and its place in the periodic table were more fully understood. The great numbers of synthetic organic chemicals that have been created since the end of World War II were not of much public interest until the publication of Rachel Carson’s book Silent Spring in 1962. This book stimulated great interest in the effects of pesticides on environmental and public health and brought to public attention the proliferation of chemicals.
The number and variety of synthetic organic chemicals are truly amazing. In 1978, the American Chemical Society’s registry of chemicals listed over 4 million organic and inorganic chemicals; of this number, more than 95 percent were organic. Of all the known organic chemicals, perhaps half are naturally occurring chemicals that have been synthesized in the laboratory or isolated from natural sources. Between 1965 and 1983, 6 million additional chemicals had been produced, and the rate of synthesis has only increased since then.
For the average person, what is the significance of the existence of these millions of chemicals? Among those that are not naturally occurring, a great many exist only in small quantities in vials on chemists’ benches or in chemical storerooms. They have not been found to have any practical use or function, and so they have not been developed commercially—yet. However, with the advent of high-throughput screening techniques where robotics speed up the screening process, many thousands of chemicals can be rapidly analyzed for their ability to bind to various animal and human chemical receptors. Out of this screening, chemicals that were once thought to have no value are being identified as potential medicines and pesticides and for other human uses.
The toxicity of synthetic chemicals—that is, the degree to which they are poisonous—covers the entire range from essentially nontoxic to extremely toxic. This is also true of inorganic compounds (think water and arsenic). Some synthetic chemicals, such as artificial sweeteners, are edible, whereas others, such as chemical warfare agents, are lethal in extremely small amounts. Regardless of the degree of toxicity, the principles of toxicology apply equally to all chemicals, whether synthetic or natural, organic or inorganic.
The number of chemicals that actually enter homes is not known, but a survey of the wide variety of products found in the home setting—such as cleansers, polishes, drugs, cosmetics, prepared foods, pesticides and other garden chemicals, automotive products, and hobby products—suggests that it is quite large. Despite the wide variety of products, many contain the same basic chemicals. Thus, the actual number of individual chemicals that the average person comes in contact with in home products is probably much closer to several thousand rather than several million. The majority of chemicals that enter homes are not harmful when used properly, but some are treated with a more cavalier attitude than is warranted, as witnessed by the numerous accidental poisonings that occur in children.
The people in contact with the widest variety of potentially dangerous chemicals are those in businesses or professions that use chemicals in some process or procedure and those who work in industries that synthesize, manufacture, formulate, or use chemicals to make other products. Few of these chemicals find their way into a home setting.
CHEMICAL CATEGORIES
We categorize chemicals in many different ways, the broadest of which is whether they are natural—produced by a living process—or synthetic—made by humans. Other ways we classify chemicals are by the use we make of them (foods, drugs, pesticides, etc.), how they are physically organized (solid, liquid, gas), what kind of animal they are (fish, reptiles, birds, mammals, etc.), whether they are organic or inorganic (animal, vegetable, or mineral), and so forth. Plant and animal probably were two of the earliest categories recognized by humans. Plants stayed put, whereas animals usually moved about freely. Based on this classification, corals were considered plants for many years until their animal nature was discovered.
A scheme of classification by the use we make of a chemical or product is essential for government regulation of such items as foods, drugs, cosmetics, pesticides, industrial chemicals, and medical devices. If a substance is claimed to be a food, it is governed by the food laws. If the exact same substance is packaged and labeled a drug, it is governed by the drug laws, not by the food laws. The laws that pertain depend on what use the manufacturer or seller specifies for the product. For example, hydrochloric acid is regulated as a household product when it is present in cleaning compounds, as a drug when it is used to treat people with low gastric acidity, as a hazardous industrial chemical when it is used in electroplating, and as a antibacterial adjuvant when it is used to enhance the germicidal activity of chlorine in swimming pools. Hydrochloric acid is natural when produced by the stomach and synthetic when made in the laboratory. Interestingly, all things tobacco are regulated by the Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF), but since 2009 the Food and Drug Administration (FDA) is monitoring the advertising and content of cigarettes, emphasizing the toxic nature of cigarette ingredients and smoke. (The ATF was originally part of the Department of the Treasury and was primarily concerned with collecting revenue generated by taxes on the items it regulated. ATF still is involved in investigating the smuggling of cigarettes.)
Another example is boric acid, which occurs naturally as the mineral sassolite but also can be synthesized in the laboratory. It is regulated as a household product when used in laundry detergents, as a drug when sold as an antiseptic eyewash, as an insecticide when used to kill roaches, as an herbicide when applied to kill weeds, and as a flame retardant when used to fireproof fabrics. Many chemicals, such as hydrochloric acid and boric acid,...
Table of contents
- Cover
- Title page
- Copyright page
- INTRODUCTION TO THE THIRD EDITION
- PREFACE TO THE SECOND EDITION
- CHAPTER 1 WHAT ARE CHEMICALS?
- CHAPTER 2 WHAT HARM DO CHEMICALS CAUSE?
- CHAPTER 3 WHAT IS TOXICOLOGY?
- CHAPTER 4 WHAT FACTORS INFLUENCE THE TOXIC EFFECTS OF CHEMICALS?
- CHAPTER 5 HOW IS TOXICOLOGY STUDIED?
- CHAPTER 6 GENERAL TOXICOLOGY
- CHAPTER 7 MUTAGENESIS AND CARCINOGENESIS
- CHAPTER 8 DEVELOPMENTAL AND REPRODUCTIVE TOXICITY
- CHAPTER 9 CASE STUDIES IN TOXICOLOGY
- CHAPTER 10 EPIDEMIOLOGY
- CHAPTER 11 THE STUDY OF RISK
- BIBLIOGRAPHY
- ABBREVIATIONS
- GLOSSARY
- APPENDIX A
- Index