
eBook - ePub
Pharmaceutical Process Chemistry for Synthesis
Rethinking the Routes to Scale-Up
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
There is a need to explain that generic versions of a drug may not be manufactured by the same process as brand-name drugs and that the different processes may have dramatically different environmental impacts. Two global forces are at odds today—the push for "greener" processes and the push for lower drug prices. This book brings this conflict into sharp focus by discussing in detail the published process chemistry for top-selling small molecule drugs.
Providing insights about process route selection, choice of reagents, and reaction conditions, Pharmaceutical Process Chemistry for Synthesis guides process chemists in identifying best processes for manufacturing these blockbuster drugs as they lose patent protection. Further, it highlights the strategies and methodology that might be useful for expediting the process research and development of the blockbusters of the future.
Written from a refreshingly objective perspective, this book is essential for process chemists who need to devise practical syntheses for increasingly complex drugs in a constantly decreasing time frame.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Pharmaceutical Process Chemistry for Synthesis by Peter J. Harrington in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
1.1 Inspiration
This project was first conceptualized at a most unlikely place: at a visit to an Inspiring Impressionism exposition at the Denver Art Museum in 2008. The exhibition focused on the impressionists as students of earlier masters. They immersed themselves in these earlier masterpieces and then incorporated the insights they had gained and added their own techniques to convey the same subject matter in profound new ways. My 20 years as a process chemist at Syntex and Roche are much like the years the impressionists spent camped out in front of the works of the masters. The insights gained could be conveyed by presenting the theory and concepts of process research and development, but there are many well-worn reference books that collectively accomplish that objective. My experience has been that process chemistry is a roller-coaster ride, with tremendous highs and lows, where you learn theory and concepts, as needed, on the fly, from your colleagues and from those reference books (while meeting seemingly unattainable milestones and timelines). The aim of this book is to convey some of this experience by immersing the reader in the process chemistry of some of the most valuable pharmaceuticals we are fortunate to have available today. The masterpieces in this book are the top-selling drugs in the United States in 2007–2008. These are Lipitor®, Nexium®, Advair Diskus®, Prevacid®, Plavix®, Singulair®, Seroquel®, Effexor XR®, Lexapro®, and Actos®, all “blockbuster” drugs, generating more than $1 billion in revenue for their owners each year (Figure 1.1).1
Figure 1.1 The top-selling drugs in the United States in 2007.
I have no previous detailed knowledge of the process chemistry of most of these drugs. Why choose these as the subject matter? First, there is currently intense interest in the process chemistry of these drugs. Second, if I had detailed unpublished knowledge about these drugs, I would be bound by a secrecy agreement to discuss only information already in the public domain. Third, having no financial stake in any of these drugs or their process technology, I can be completely (and refreshingly) objective. I am not “selling” the value of any target or proprietary technology to a patent agency or a pharmaceutical manufacturer.
After a detailed review of the process chemistry for Plavix® and Nexium®, these will not be included. The process chemistry for Plavix® is omitted because I have published and patented process work and have detailed knowledge of the manufacturing process for Ticlid®. The antiplatelet drug Ticlid® is an adenosine diphosphate (ADP) receptor inhibitor with the same thienopyridine core as Plavix® (Figure 1.2).2 The process chemistry for Nexium® is omitted because Prevacid® and Nexium® have the same core and there is considerable overlap in their process chemistry. Advair Diskus® has two active ingredients: salmeterol and fluticasone. The process chemistry of salmeterol is included. The process chemistry of fluticasone would be better presented “in context” with the process chemistry of other valuable steroids.
Figure 1.2 The close structure similarity between the antiplatelet drugs Plavix® and Ticlid®.
With this format, will this book touch on every important aspect of process chemistry in the pharmaceutical industry? If you carefully studied the techniques used to create 10 masterpieces at the art museum would you become an art expert? Most people would say no. Would you be better able to utilize the techniques in your own paintings? Most people would say yes. The scientific objective of this book is then twofold: to identify one “best” process for manufacturing these blockbuster drugs and to highlight the strategies and methodology that might be useful for expediting the process research and development of the blockbusters of the future.
1.2 Information Sources
This project must begin with meaningful and realistic objectives. A consistent strategy will be used to define, retrieve, and review the relevant literature. The process chemistry presented is based on published experimental data harvested from patents and journal publications. The majority of the information is taken from U.S., European (EP), and World (WO) patents. Other country-specific patents are included if they are cross-referenced several times, do not have a U.S./EP/WO equivalent, and are available in English, French, or German. Working with a finite production budget, information from Chinese (CN) and Japanese (JP) patents is taken from Chemical Abstracts. Journal articles are often published in tandem with patents and offer the same experimental procedures and data. Key journal articles offering information not found in the patent literature are included. The presentation is weighted to emphasize the process patents and publications and the marketplace information published in the past decade.
It is likely that at least a few details of the process chemistry of a valuable pharmaceutical may be carefully guarded as a trade secret. Speculation about unavailable data will be clearly marked as such. Legal questions such as who owns a particular patented process, how long they will own it, or how valid are their patent claims are important questions that should be directed to a legal expert. The answers to these questions are outside the scope of this book.
A quick SciFinder® search (January 1, 2009) for the Prevacid® structure, for example, revealed approximately 1700 references. A review using this number of references for each target cannot be accomplished in a realistic time frame. A solution to this is to structure search for the building blocks unique to each target. The building blocks selected for Prevacid® are shown in Figure 1.3. The building block structure searches provide the first generation of references. The cross-references from the first generation are then used and the process repeated until the cross-reference loop is completed. For Prevacid®, this structure search approach reduced 1700 references to a manageable 200 references. The structures searched are provided at the end of each chapter. No effort was made to update the chapters completed first.
Figure 1.3 Building blocks searched to provide references to process chemistry for Prevacid®.
Process chemistry is so multidimensional that there will inevitably be important points overlooked. I welcome your comments and suggestions for improving the content and format of future publications.
1.3 Content and Format for Presentation
The content of each chapter will vary according to the information harvested from the references. For example, one chapter emphasizes the manufacturing route selection while another focuses on conversion of the penultimate intermediate to the final target. This variable content accurately reflects the range of tasks assigned to process chemists. Your role in a process research and development team may be early route selection in one project. Your role may be late troubleshooting of a difficult crystallization to produce a target that filters well and meets crystal size and purity specifications in another. Your role might involve working closely with procurement specialists or engineers in the early route selection or with analytical and regulatory specialists on the difficult crystallization.
Just as the chemical transformations are central to the manufacturing process, the process chemist is the hub of manufacturing process research and development. The process chemist does not have to be an expert in the related specialties of marketing strategy, patent law, procurement, environmental health and safety, analytical chemistry, formulation, regulatory affairs, and engineering and facilities but he must be knowledgeable enough to identify questions best answered working in close collaboration with these experts. Answers will sometimes be offered to questions best answered by these experts with the understanding that the answer is meant to trigger a discussion with the expert.
Each chapter is written to stand alone. Chapters 2, 3, 4, 5, 6, 7, 8, 9 can be read in any order. While the content for each chapter will vary, the same format will be used to present the available information. Each chapter begins with an overview of current and past marketplace information for the target. This discussion is included to emphasize that the process research and development team cannot work in a vacuum. The team should receive detailed updates at regular intervals on the market potential of the target, the timing of the delivery, and new clinical and post-launch data that may impact the market potential and timing of the delivery. This information might come from a marketing or business development expert.
To minimize repetition, retrosynthetic analysis will not be used to stage the synthesis discussion. To emphasize the modularity of pharmaceutical manufacturing, the synthesis discussion in each chapter starts with identification of raw materials. These raw materials are usually commercially available or can be produced in a few steps from commercial materials.
Every process begins with commercially available raw materials. A price is provided for each raw material that contributes at least one atom to the target when that raw material first appears in the discussion. Since suppliers and prices for raw materials are in constant flux, all prices quoted are taken from the 2007–2008 Aldrich catalog. It is my intention that these prices will give a “snapshot” of a relative price and availability at this point in time. Quoting an Aldrich catalog price should suggest scheduling a preliminary communication with a procurement group. This communication would include estimates of the quantity and purity specifications, a preferred delivery date, and any special shipping and handling requirements. Other raw materials, for example, acids, bases, reagents used to create protecting groups or leaving groups, drying agents, filter aids, and decolorizing carbon are not priced since expensive materials might be replaced by less expensive alternatives.
The raw material prices are only intended for “back-of-the-envelope” calculations. Detailed cost calculations should include vendor-guaranteed raw material prices and labor and overhead (LOH) costs for the manufacturing site and are beyond the scope of this book.
Aldrich catalog names are used for all starting materials and ChemDraw 11.0® is used to generate names for all process intermediates. With the intention that each sentence can stand alone, full chemical names are used in the text in many cases. Process intermediates and products are each assigned a number to facilitate correlation of the names with the structures in schemes and figures. An example of a stand-alone sentence is taken from the Seroquel® discussion.
The reaction of 11-chlorodibenzo[b,f][1,4]thiazepine (25) with 2-(2-(piperazin-1-yl)ethoxy)ethanol (26) (2.0 equivalents) in refluxing toluene is complete in 8 h.
Patent procedures often contain data gaps. These can be separated into two categories. A major data gap is missing information that would certainly have been generated but was not included in the process description. Examples of major data gaps are a missing quantity for one reagent of several or a missing volume for the reaction solvent. Major data gaps are clearly identified in the discussion, and where possible, an attempt is made to fill the gaps with information gleaned from another source. A minor data gap is information presented in a format that requires a translation. For example, reagent quantities might be quoted only in weights or volumes. This gap is filled by converting reagent quantities into equivalents. In process chemistry, an equivalent simply refers to the number of moles of reagent per mole of limiting reagent. Equivalents in this book are calculated to the nearest 0.1.
Solvents and reaction temperatures are critically important process characteristics. These are included in each reaction description. After selecting a best process, the process solvents used are revisited to emphasize the importance of minimizing the number of process solvents and to highlight the solvents commonly used in a pharmaceutical manufacturing plant. Temperatures in the range of 20–30°C, or “ambient,” are standardized as 25°C in the reaction descriptions. Very low temperatures (<−70°C) require that expensive liquid nitrogen be available locally and that liquid nitrogen storage facilities be available on site. Expensive circulating fluid and energy are required to achieve and maintain very high reaction temperatures (>160°C). Examples of a reaction description and a process solvent review are taken from the Actos® discussion.
The condensation of 4-(2-(5-ethylpyridin-2-yl)ethoxy)benzaldehyde (19) with thiazolidine-2,4-dione (1.2 equivalents) and pyrrolidine (1.0 equivalent) in methanol at 45°C is very efficient even after multiple precipitations and isolations for purity upgrade (95% yield). The process solvents are toluene, THF, ethanol, isopropanol, and water, all solvents commonly used in a pharmaceutical manufacturing plant.
It is assumed that all operations involving combustible organic materials are performed under nitrogen and that all chemical mixtures are stirred. This is not specifically stated in the procedures described.
When there are many similar procedures, they will be presented in a parallel format to facilitate comparison and highlight the differences. Material presented in parallel format is usually preceded by a summary of the trends and results. An example of parallel formatting is taken from the Effexor XR® discussion.
A mixture...
Table of contents
- Cover
- Title Page
- Copyright
- Chapter 1: Introduction
- Chapter 2: Actos® (Pioglitazone Hydrochloride)
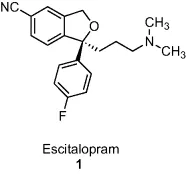
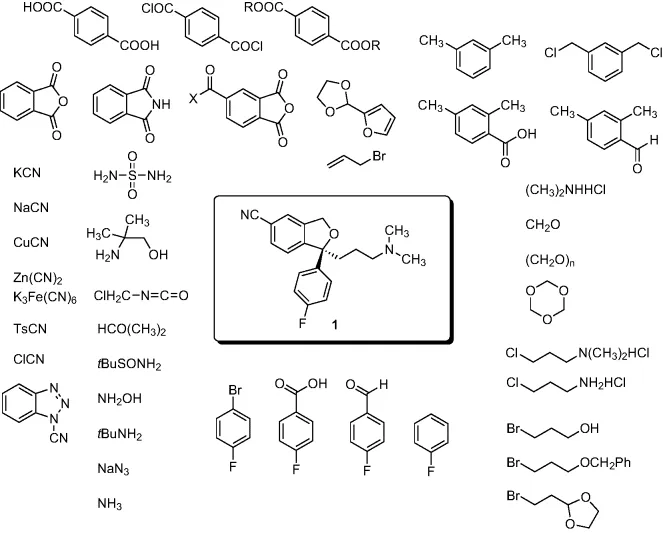
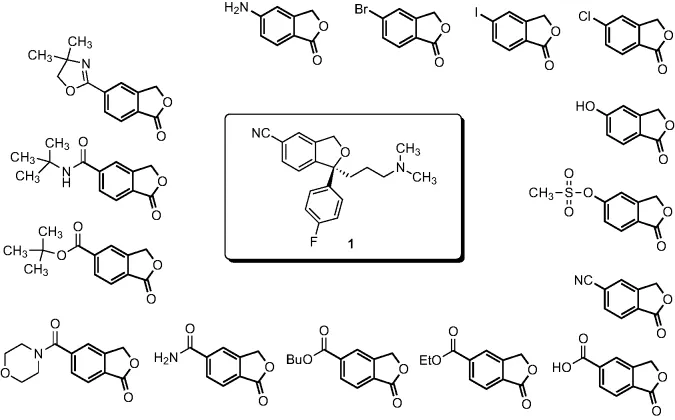
- Chapter 3: Lexapro® (Escitalopram Oxalate)
- Chapter 4: Effexor XR® (Venlafaxine Hydrochloride)
- Chapter 5: Seroquel® (Quetiapine Hemifumarate)
- Chapter 6: Singulair® (Montelukast Sodium)
- Chapter 7: Prevacid® (Lansoprazole)
- Chapter 8: Advair Diskus® (Salmeterol Xinafoate)
- Chapter 9: Lipitor® (Atorvastatin Calcium)
- Index