
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Biological Chemistry of Arsenic, Antimony and Bismuth
About this book
Arsenic, antimony and bismuth, three related elements of group 15, are all found in trace quantities in nature and have interesting biological properties and uses. While arsenic is most well known as a poison - and indeed the contamination of groundwater by arsenic is becoming a major health problem in Asia - it also has uses for the treatment of blood cancer and has long been used in traditional chinese medicine. Antimony and bismuth compounds are used in the clinic for the treatment of parasitic and bacterial infections.
Biological Chemistry of Arsenic, Antimony and Bismuth is an essential overview of the biological chemistry of these three elements, with contributions from an international panel of experts. Topics covered include:
- chemistry of As, Sb and Bi
- biological chemistry of arsenic
- biological chemistry of Sb and Bi
- arsenic and antimony speciation in environmental and biological samples
- arsenic in traditional chinese medicine
- arsenic in aquifers
- biomethylation of As, Sb and Bi
- uptake of metalloids by cells
- bismuth complexes of porphyrins and their potential in medical applications
- Helicobacter pylori and bismuth
- metabolism of arsenic trioxide in blood of the acute promyelocytic leukemia patients
- anticancer properties of As, Sb and Bi
- radio-Bi in cancer therapy
- genotoxicity of As, Sb and Bi
- metallomics as a new technique for As, Sb and Bi
- metalloproteomics for As, Sb and Bi
Biological Chemistry of Arsenic, Antimony and Bismuth conveys the essential aspects of the bioinorganic chemistry of these three elements, making this book a valuable complement to more general bioinorganic chemistry texts and more specialized topical reviews. It will find a place on the bookshelves of practitioners, researchers and students working in bioinorganic chemistry and medicinal chemistry.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Biological Chemistry of Arsenic, Antimony and Bismuth by Hongzhe Sun in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biochemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
The Chemistry of Arsenic, Antimony and Bismuth
Arsenic, antimony and bismuth are the heavier pnictogen (Group 15) elements and consistent with their lighter congeners, nitrogen and phosphorus, they adopt the ground state electron configuration ns2np3. Arsenic and antimony are considered to be metalloids and bismuth is metallic, while nitrogen and phosphorus are non-metals. Arsenic and antimony are renowned for their toxicity or negative bioactivity [1, 2] but bismuth is well known to provide therapeutic responses or demonstrate a positive bioactivity [3]. As a background to the biological and medicinal chemistry of these elements, the fundamental chemical properties of arsenic, antimony and bismuth are presented in this introductory chapter.
1.1 Properties of the Elements
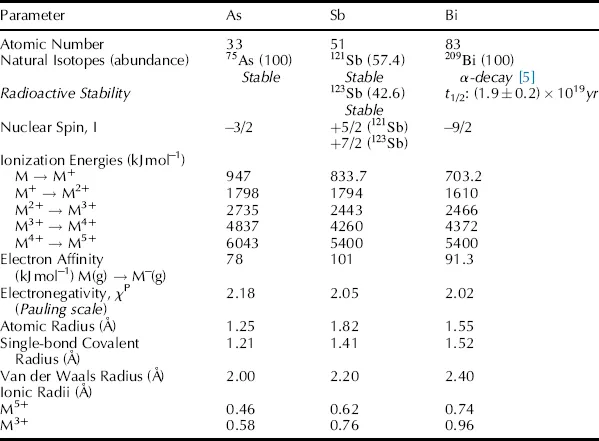
Selected fundamental parameters that define the heavier pnictogen elements are summarized in Table 1.1 [4]. While arsenic and bismuth are monoisotopic, antimony exists as two substantially abundant naturally occurring isotopes. All isotopes of the heavy pnictogens are NMR active nuclei, indicating that the nuclear spin will interact with an applied magnetic field. However, as the nuclear spins of these isotopes are all quadrupolar, NMR spectra generally consist of broad peaks and provide limited information. The atoms As, Sb and Bi all have the same effective nuclear charge (Zeff = 6.30, Slater), which estimates the charge experienced by a valence electron taking into account shielding by the other electrons. As a consequence, the ionization energies and electron affinities for As, Sb and Bi are very similar. The ionization energy is the energy required to remove a valence electron from an atom or an ion in the gas phase. The ionization energies are predictably greater for ions with higher positive charge and are typically lower for atoms or ions with higher principal quantum number (n). The electron affinity is the energy released when an atom gains an electron to form an anion in the gas phase. The electronegativity (χP), defining the relative ability of an atom to attract electrons to itself in a covalent bond, is sufficiently larger for arsenic than for antimony and bismuth. The atomic radii, covalent radii and ionic radii are smallest for arsenic and largest for bismuth atoms consistent with the relative atomic mass and number of electron shells.
Table 1.1 Elemental parameters for arsenic, antimony and bismuth (adapted with permission from [4]). Copyright Springer Science + Business Media
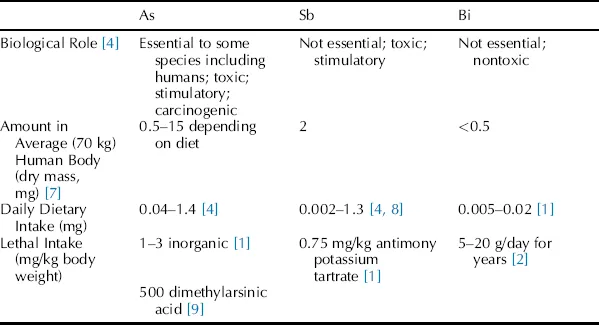
Selected biological and toxicity data for As, Sb and Bi are summarized in Table 1.2. While some arsenic compounds are essential to certain animal species [4], most arsenic compounds display toxic biological effects even when present in only small amounts. Some compounds, such as Salvarsan 606 [6], are therapeutic, although there are reported side effects, including death in high dosages. Neither antimony nor bismuth has any known natural biological function. While antimony has toxicity comparable with that of arsenic, bismuth can be tolerated in large quantities. Bismuth compounds have been used for more than two centuries to treat many medical disorders and are now commonly available in the preparations known commercially as Peptobismol and DeNol [3].
Table 1.2 Biological and toxicity data for arsenic, antimony and bismuth
1.2 Allotropes
Elemental antimony and bismuth are most stable in the αform, which is rhombohedral and typically grey in appearance, while the most common form of arsenic is β-arsenic (grey arsenic). The α-allotropic forms are analogous to black phosphorus, composed of layers of hexagonally connected sheets, as shown in Figure 1.1. The interatomic distances (r1, r2) are predictably larger for the heavier elements due to their larger atomic radii. The difference in the interlayer distance (r2) between each adjacent pnictogen atom decreases from P to As to Sb to Bi (Table 1.3).
Figure 1.1 Schematic drawing of the α-rhombohedral form of elemental As, Sb or Bi, r 1 is the interatomic distance within a sheet and r2 is the interlayer distance (Table 1.3)
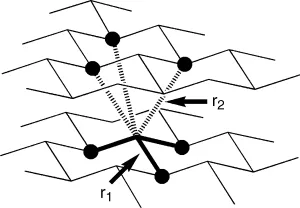
Table 1.3 Comparative structural parameters for α-rhombohedral arsenic, antimony and bismuth at 298 K

Arsenic is observed to exist in two (yellow and black) [10, 11] additional allotropic forms, while antimony adopts five allotropes [11, 12] and bismuth adopts at least three allotropes [11]. Most of these alternate allotropes are only nominally stable or require high temperature or pressure conditions [11, 13].
1.3 Bond Energies
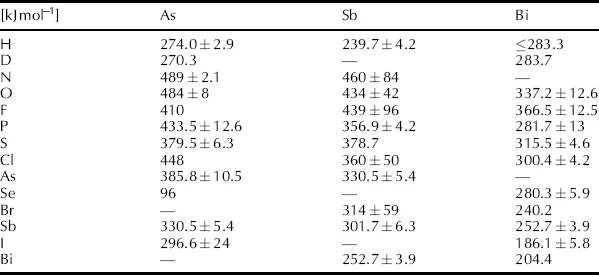
Arsenic, antimony and bismuth form stable covalent bonds with most elements. For direct comparison, Table 1.4 lists experimentally determined bond energies for the dissociation of selected pnictogen-element diatomic species in the gas phase. While these energies are not representative of pnictogen element bonds in larger molecules, the same relative trends are exhibited. Bond energies are dependent on the molecular environment in the specific compound studied. For a particular element, Pn-element bond energies generally decrease from As to Sb to Bi. For example, the Pn–H bonds in AsH3 and SbH3 are 319.2 kJ mol−1 and 288.3 kJ mol−1, respectively [18]. Moreover, bonds involving lighter elements are generally stronger. For example, the Bi–X bonds in BiF3 and BiBr3 are 435 kJ mol−1 and 297.1 kJ mol−1, respectively [18]. Similarly, in OAsPh3 and SAsPh3, the As=Ch bond is 429 kJ mol−1 and 293 kJ mol−1, for Ch=O and Ch=S respectively [18].
Table 1.4 Experimentally determined pnictogen-element bond energies of selected diatomic molecules in the gas phase, kJ mol−1 (from reference [19])
1.4 Oxidation States
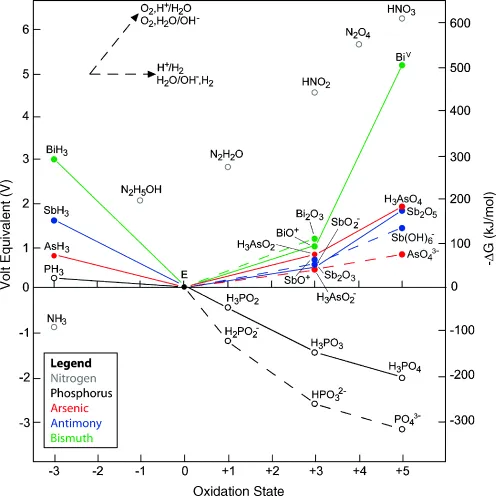
The pnictogen elements access oxidation states ranging from −3 to +5, as summarized in Figure 1.2, which presents the relative energy of each oxidation state in volts (J C−1) and in Gibbs energy. In contrast to nitrogen and phosphorus, arsenic, antimony and bismuth thermodynamically favour the elemental form. While positive oxidation states for phosphorus are stable, compounds containing arsenic, antimony or bismuth in positive oxidation states are unstable with respect to elemental forms. This phenomenon is most dramatic when comparing the relative energy differences for compounds containing pnictogens in +5 oxidation state.
Figure 1.2 Oxidation state diagram for the pnictogen elements. Dashed lines represent basic conditions. Solid lines represent acidic conditions. Adapted with permission from [11]. Copyright Elsevier (1997)
1.5 Relativistic Effects and Orbital Contraction
The property trends observed for the pnictogens can be rationalized by consideration of orbital contr...
Table of contents
- Cover
- Title Page
- Copyright
- List of Contributors
- Preface
- Chapter 1: The Chemistry of Arsenic, Antimony and Bismuth
- Chapter 2: Arsenic's Interactions with Macromolecules and its Relationship to Carcinogenesis
- Chapter 3: Biological Chemistry of Antimony and Bismuth
- Chapter 4: Metallomics Research Related to Arsenic
- Chapter 5: Arsenic in Traditional Chinese Medicine
- Chapter 6: Microbial Transformations of Arsenic in Aquifers
- Chapter 7: Biomethylation of Arsenic, Antimony and Bismuth
- Chapter 8: Metalloid Transport Systems
- Chapter 9: Bismuth Complexes of Porphyrins and their Potential in Medical Applications
- Chapter 10: Helicobacter pylori and Bismuth
- Chapter 11: Application of Arsenic Trioxide Therapy for Patients with Leukaemia
- Chapter 12: Anticancer Activity of Molecular Compounds of Arsenic, Antimony and Bismuth
- Chapter 13: Radiobismuth for Therapy
- Chapter 14: Genetic Toxicology of Arsenic and Antimony
- Chapter 15: Metalloproteomics of Arsenic, Antimony and Bismuth Based Drugs
- Index