
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Heterocyclic Chemistry At A Glance
About this book
This expanded second edition provides a concise overview of the main principles and reactions of heterocyclic chemistry for undergraduate students studying chemistry and related courses. Using a successful and student-friendly "at a glance" approach, this book helps the student grasp the essence of heterocyclic chemistry, ensuring that they can confidently use that knowledge when required. The chapters are thoroughly revised and updated with references to books and reviews; extra examples and student exercises with answers online; and color diagrams that emphasize exactly what is happening in the reaction chemistry depicted.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Heterocyclic Chemistry At A Glance by John A. Joule,Keith Mills in PDF and/or ePUB format, as well as other popular books in Scienze fisiche & Chimica organica. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Heterocyclic Nomenclature
A selection of the structures, names and standard numbering of the more common heteroaromatic systems and some common non-aromatic heterocycles, are shown in this chapter. The aromatic heterocycles are grouped into those with six-membered rings and those with five-membered rings. The names of six-membered aromatic heterocycles that contain nitrogen generally end in ‘ine’, though note that ‘purine’ is the name for a very important bicyclic system which, has both a six- and a five-membered nitrogen-containing heterocycle. Five-membered heterocycles containing nitrogen generally end with ‘ole’. Note the use of italic ‘H’ in a name such as ‘9H-purine’ to designate the location of an N-hydrogen in a system in which, by tautomerism, the hydrogen could reside on another nitrogen (e.g. N-7 in the case of purine). Names such as ‘pyridine’, ‘pyrrole’ and ‘thiophene’ are the original, and now standard, names for these heterocycles; names such as ‘1,2,4-triazine’ for a six-membered ring with three nitrogens located as indicated by the numbers, are more logically systematic.
A detailed discussion of the systematic rules for naming polycyclic systems in which several aromatic or heteroaromatic rings are fused together, is beyond the scope of this book, however, two simple examples will serve to illustrate the principles. In the name ‘pyrrolo[2,3-b]pyridine’, the numbers signify the positions of the first named heterocycle, numbered as if it were a separate entity, which are the points of ring fusion; the italic letter, ‘b’ in this case, designates the side of the second named heterocycle to which the other ring is fused, the lettering deriving from the numbering of that heterocycle as a separate entity, that is, side a is between atoms 1 and 2, side b is that between atoms 2 and 3, and so on. Actually, this particular heterocycle is more often referred to as ‘7-azaindole’ – note the use of the prefix ‘aza’ to denote the replacement of a ring carbon by nitrogen. Similarly, ‘5-azaindole’ is systematically called ‘pyrrolo[3,2-c]pyridine’ – note that the order of the numbers ‘3,2-’ arises because the first atom of the pyrrole encountered in counting round from the pyridine nitrogen to determine the side of fusion, and thus the label ‘c’, is C-3 of the pyrrole unit. The numbering of a bi- or polycyclic system as a whole is generated from a series of rules concerned with the orientation of the rings and the positions of the nitrogen(s), but we do not deal with these here – the overall numbering for these two systems is shown for two substituted examples.
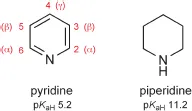
A device that is useful in discussions of reactivity is the designation of positions as ‘α’, ‘β’ or ‘γ’. For example, the 2- and the 6-positions in pyridine are equivalent in reactivity terms, so to make discussion of such reactivity clearer, each of these positions is referred to as an ‘α-position’. Comparable use of α and β is made in describing reactivity in five-membered systems. These useful designations are shown on some of the structures. Note that carbons at angular positions do not have a separate number but are designated using the number of the preceding atom followed by ‘a’ – as illustrated for quinoline.
Six-Membered Aromatic Heterocycles
Five-Membered Aromatic Heterocycles
Non-Aromatic Heterocycles
Small-Ring Heterocycles
Chapter 2
Structures of Heteroaromatic Compounds
Structures of Benzene and Naphthalene
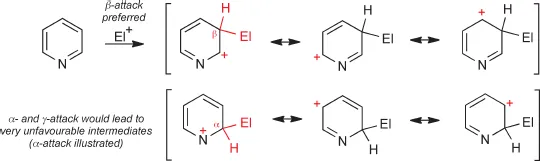
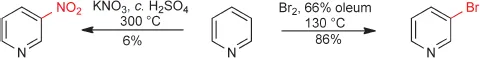
We start our consideration of heteroaromatic structures by recalling the prototypical structures of aromatic hydrocarbons such as benzene and naphthalene. Hückel's rule states that aromaticity is associated with fully conjugated cyclic systems of 4n+2 π-electrons, that is with 2, 6, 10, 14 and so on, π-electrons, with 6π-electron monocyclic compounds being by far the commonest. Thus, benzene has a cyclic arrangement of six π-electrons comprising a conjugated molecular orbital system that is thermodynamically much more stable than a corresponding non-cyclically conjugated system – this additional stabilisation is called ‘resonance energy’ and has a value of about 152 kJ mol1 for benzene. Compared with alkenes, this results in a much diminished tendency to react with electrophiles by addition and a greater tendency to react by substitution of hydrogen. Addition reactions would lead to products in which a substantial proportion of the resonance energy had been lost. As we shall remind ourselves in Chapter 3, electrophilic substitution is the prototypical reaction of benzene.

In benzene, the geometry of the ring, with angles of 120°, fits precisely the geometry of a planar trigonally hybridised carbon atom, and allows the assembly of a σ-skeleton of six sp2 hybridised carbon atoms in a strainless planar ring. Each carbon then has one extra electron, which occupies an atomic p orbital orthogonal to the plane of the ring. The p orbitals interact sideways to generate the π-molecular orbitals associated with the aromatic system.
We shall represent the stabilising delocalisation of aromatic molecules by drawing ‘mesomeric structures’, thus benzene is represented as a ‘resonance hybrid’ of the two extreme forms. These have no existence in their own right, but are ‘resonance contributors’ to the ‘real’ structure. The use of mesomeric structures is particularly useful in representing the polarisation inherent in many heterocycles and, especially, for representing the delocalisation of charge in reaction intermediates. We shall find them invaluable in helping to understand heteroaromatic reactivity and regioselectivity.

Naphthalene, with ten carbons and ten orthogonal p orbitals, has an aromatic system with ten π-electrons. Naphthalene is represented by three mesomeric structures and has a resonance energy of about 255 kJ mol-1, substantially less than twice that of benzene.
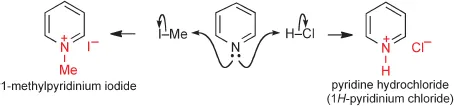
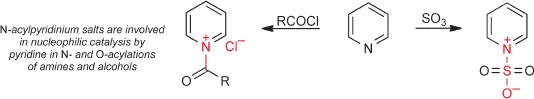
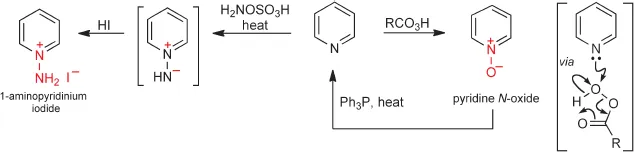
Structures of Pyridines and Pyridiniums
The structure of pyridine is completely analogous to that of benzene, being related through replacement of CH by N. The key differences a...
Table of contents
- Cover
- Other Titles Available in the Chemistry at a Glance series:
- Title Page
- Copyright
- Biography
- Abbreviations
- Introduction to Second Edition
- Chapter 1: Heterocyclic Nomenclature
- Chapter 2: Structures of Heteroaromatic Compounds
- Chapter 3: Common Reaction Types in Heterocyclic Chemistry
- Chapter 4: Palladium in Heterocyclic Chemistry
- Chapter 5: Pyridines
- Chapter 6: Diazines
- Chapter 7: Quinolines and Isoquinolines
- Chapter 8: Pyryliums, Benzopyryliums, Pyrones and Benzopyrones
- Chapter 9: Pyrroles
- Chapter 10: Indoles
- Chapter 11: Furans and Thiophenes
- Chapter 12: 1,2-Azoles and 1,3-Azoles
- Chapter 13: Purines
- Chapter 14: Heterocycles with More than Two Heteroatoms: Higher Azoles (5-Membered) and Higher Azines (6-Membered)
- Chapter 15: Heterocycles with Ring-Junction Nitrogen (Bridgehead Nitrogen)
- Chapter 16: Non-Aromatic Heterocycles
- Chapter 17: Heterocycles in Nature
- Chapter 18: Heterocycles in Medicine
- Chapter 19: Applications and Occurrences of Heterocycles in Everyday Life
- Index