
Stephens' Detection and Evaluation of Adverse Drug Reactions
Principles and Practice
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Stephens' Detection and Evaluation of Adverse Drug Reactions
Principles and Practice
About this book
Stephens' Detection and Evaluation of Adverse Drug Reactions provides a comprehensive review of all aspects of adverse drug reactions throughout the life cycle of a medicine, from toxicology and clinical trials through to pharmacovigilance, risk management, and legal and regulatory requirements. It also covers the safety of biotherapeutics and vaccines and includes new chapters on pharmacogenetics, proactive risk management, societal considerations, and the safety of drugs used in oncology and herbal medicines.
This sixth edition of the classic text on drug safety is an authoritative reference text for all those who work in pharmacovigilance or have an interest in adverse drug reactions, whether in regulatory authorities, pharmaceutical companies, or academia.
Praise for previous editions
"This book presents a comprehensive and wide-ranging overview of the science of pharmacovigilance. For those entering or already experienced in the pharmaceutical sciences, this is an essential work." - from a review in E-STREAMS
"...a key text in the area of pharmacovigilance...extensively referenced and well-written...a valuable resource..." - from a review in The Pharmaceutical Journal
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
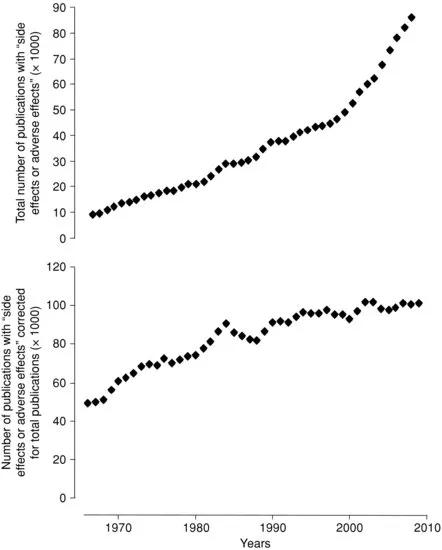
- the identification and quantification of previously unrecognized adverse effects and reactions;
- the identification of subgroups of patients at particular risk of adverse reactions;
- the continued surveillance of a product throughout the duration of its use, to ensure that the balance of its benefits and harms are and remain acceptable;
- the description of the comparative adverse reactions profile of products within the same therapeutic class;
- the detection of inappropriate prescription and administration;
- the further elucidation of a product's pharmacological and toxicological properties and the mechanism(s) by which it produces adverse effects;
- the detection of clinically important drug–drug, drug–herb/herbal medicine, drug–food, and drug–device interactions;
- the communication of appropriate information to health-care professionals;
- the confirmation or refutation of false-positive signals that arise, whether in the professional or lay media, or from spontaneous reports.
Table of contents
- Cover
- Title Page
- Copyright
- Foreword
- Preface to the Sixth Edition
- Contributors
- Acknowledgements
- 1: Adverse Drug Reactions: History, Terminology, Classification, Causality, Frequency, Preventability
- 2: Pharmacogenetics of Adverse Drug Reactions
- 3: Toxicology and Adverse Drug Reactions
- 4: Clinical Trials—Collecting Safety Data and Establishing the Adverse Drug Reactions Profile
- 5: Clinical Laboratory Safety Data
- 6: Statistics: Analysis and Presentation of Safety Data
- 7: Proactive Pharmacovigilance and Risk Management
- 8: Regulatory Aspects of Pharmacovigilance
- 9: Legal Aspects of Pharmacovigilance in the European Union
- 10: Dictionaries and Coding in Pharmacovigilance
- 11: Adverse Drug Reactions: Societal Considerations
- 12: Safety of Biotherapeutics
- 13: Vaccine Safety Surveillance
- 14: Assessing the Safety of Drugs Used in Oncology
- 15: Adverse Drug Reactions and Pharmacovigilance of Herbal Medicines
- Appendix 1: Web Sites Relevant to Pharmacovigilance—An Analysis of Contents
- Appendix 2: Guidelines and a Checklist for Reporting Suspected Adverse Drug Reactions Anecdotally in Journals
- Index