
Handbook of Hot-dip Galvanization
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Handbook of Hot-dip Galvanization
About this book
Based on the successful German version, this edition has been adapted to include international standards, regulations and best practices. The book systematically covers all steps in hot-dip galvanization: surface pre-treatment, process and systems technology, environmental issues, and quality management. As a result, the reader finds the fundamentals as well as the most important aspects of process technology and technical equipment, alongside contributions on workpiece requirements for optimal galvanization results and methods for applying additional protective coatings to the galvanized pieces.
With over 200 illustrated examples, step-by-step instructions, presentations and reference tables, this is essential reading for apprentices and professionals alike.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
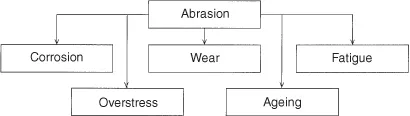
- Corrosion system: A system consisting of one or several metals and such parts of the environment that affect corrosion.
- Corrosion phenomenon: Modification in any part of the corrosion system caused by corrosion.
- Corrosion damage: Corrosion phenomenon causing the impairment of the metal function, of the environment or of the technical system of which they form a part.
- Corrosion failure: Corrosion damage characterized by the complete loss of operational capability of the technical system.
- Corrosion resistance: Ability of a metal to maintain operational capability in a given corrosion system.
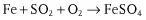
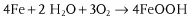
- chemical corrosion Corrosion excluding electrochemical reaction,
- electrochemical corrosion Corrosion including at least one anodic and one cathodic reaction.
- Uniform surface corrosion General corrosion occurring on the entire surface at nearly the same rate.
- Shallow pit corrosion Corrosion with locally different abrasion rates; caused by the existence of corrosion elements.
- Pitting corrosion Local corrosion resulting in holes, that is, in cavities expanding from the surface to the inside of the metal.
- Crevice corrosion Local corrosion in connection with crevices occurring in or immediately adjacent to the crevice area, which has developed between the metal surface and another surface (metal or nonmetal).
- Contact corrosion (aka dissimilar metal corrosion) Occurs at contact surfaces of different metals; the acceleratedly corroding metal area is the anode of the corrosion element.
- Intergranular corrosion Corrosion in or adjacent to the grain boundaries of a metal.
- Uniform surface attack A form of corrosion where the metal material is almost uniformly removed from the surface. This form is also the basis for the calculation of the mass loss (g m−2) or the determination of the corrosion rate (µm y−1).
- Shallow pit formation A form of corrosion with irregular surface attack forming pits with diameters much larger than their depth.
- Pitting A form of corrosion with crater-shaped or surface-excavating pits or pits resembling pin pricks. The depth of the pitting spots usually exceeds their diameter.
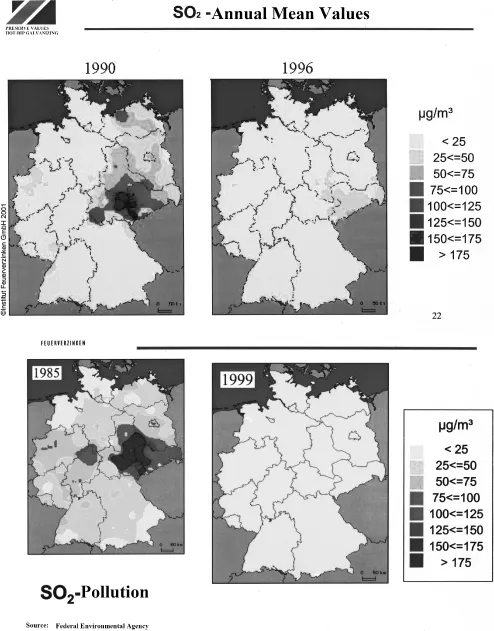
- With increasing relative humidity.
- With condensate occurring (surface temperature < dew point).
- In the presence of precipitation.
- With increasing pollution of the atmosphere which may affect the steel surface and/or be deposited on it. Pollutants are gases, including sulfur dioxide, salts, chlorides, and sulfates. In connection with humidity, deposits like soot, dusts, salts, etc., on steel surfaces accelerate corrosion.
- climatic zone;
- cold climate;
- moderate climate;
- dry climate;
- warm, humid climate;
- sea climate;
- local climate.
- atmospheric types;
- room atmosphere;
- rural atmosphere;
- urban atmosphere;
- industrial atmosphere;
- marine atmosphere;
- microclimate.
Table of contents
- Cover
- Related Titles
- Title page
- Copyright page
- Preface to the Third German Edition
- Acknowledgment
- Preface to the Second German Edition
- List of Contributors
- 1 Corrosion and Corrosion Protection
- 2 Historical Development of Hot-dip Galvanizing
- 3 Surface-preparation Technology
- 4 Hot-dip Galvanizing and Layer-formation Technology
- 5 Technical Equipment
- 6 Environmental Protection and Occupational Safety in Hot-dip Galvanizing Plants
- 7 Design and Manufacturing According to Hot-dip Galvanizing Requirements
- 8 Quality Management in Hot-dip Galvanizing Companies
- 9 Corrosion Behavior of Zinc Coatings
- 10 Coatings on Zinc Layers – Duplex-Systems
- 11 Economic Efficiency of Hot-dip Galvanizing
- 12 Examples of Use
- 13 Appendix
- Index