
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Workbook for Organic Synthesis: The Disconnection Approach
About this book
Workbook for Organic Synthesis: The Disconnection Approach, 2nd Edition
This workbook provides a comprehensive graded set of problems to illustrate and develop the themes of each of the chapters in the textbook Organic Synthesis: The Disconnection Approach, 2nd Edition. Each problem is followed by a fully explained solution and discussion. The examples extend the student's experience of the types of molecules being synthesised by organic chemists, and the strategies they employ to control their syntheses. By working through these examples students will develop their skills in analysing synthetic challenges, and build a toolkit of strategies for planning new syntheses. Examples are drawn from pharmaceuticals, agrochemicals, natural products, pheromones, perfumery and flavouring compounds, dyestuffs, monomers, and intermediates used in more advanced synthetic work. Reasons for wishing to synthesise each compound are given. Together the workbook and textbook provide a complete course in retrosynthetic analysis.
Organic Synthesis: The Disconnection Approach, 2nd Edition
There are forty chapters in Organic Synthesis: The Disconnection Approach, 2nd Edition: those on the synthesis of given types of molecules alternate with strategy chapters in which the methods just learnt are placed in a wider context. The synthesis chapters cover many ways of making each type of molecule starting with simple aromatic and aliphatic compounds with one functional group and progressing to molecules with many functional groups. The strategy chapters cover questions of selectivity, protection, stereochemistry, and develop more advanced thinking via reagents specifically designed for difficult problems. In its second edition updated examples and techniques are included and illustrated additional material has been added to take the student to the level required by the sequel, Organic Synthesis: Strategy and Control. Several chapters contain extensive new material based on courses that the authors give to chemists in the pharmaceutical industry.
Workbook for Organic Synthesis: The Disconnection Approach, 2nd edition, combined with the main textbook, provides a full course in retrosynthetic analysis for chemistry and biochemistry students, and a refresher course for organic chemists working in industry and academia.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
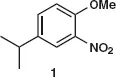
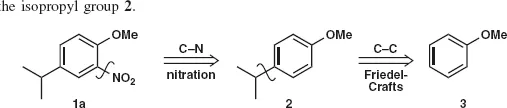
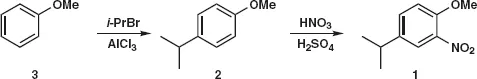
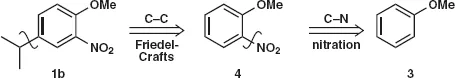
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- General References
- 1: The Disconnection Approach
- 2: Basic Principles: Synthons and Reagents: Synthesis of Aromatic Compounds
- 3: Strategy I: The Order of Events
- 4: One-Group C–X Disconnections
- 5: Strategy II: Chemoselectivity
- 6: Two-Group C–X Disconnections
- 7: Strategy III: Reversal of Polarity, Cyclisations, Summary of Strategy
- 8: Amine Synthesis
- 9: Strategy IV: Protecting Groups
- 10: One-Group C–C Disconnections I: Alcohols
- 11: General Strategy A: Choosing a Disconnection
- 12: Strategy V: Stereoselectivity A
- 13: One-Group C–C Disconnections II: Carbonyl Compounds
- 14: Strategy VI: Regioselectivity
- 15: Alkene Synthesis
- 16: Strategy VII: Use of Acetylenes (Alkynes)
- 17: Two-Group C–C Disconnections I: Diels-Alder Reactions
- 18: Strategy VIII: Introduction to Carbonyl Condensations
- 19: Two-Group C–C Disconnections II: 1,3-Difunctionalised Compounds
- 20: Strategy IX: Control in Carbonyl Condensations
- 21: Two-Group C–C Disconnections III: 1,5-Difunctionalised Compounds Conjugate (Michael) Addition and Robinson Annelation
- 22: Strategy X: Aliphatic Nitro Compounds in Synthesis
- 23: Two-Group Disconnections IV: 1,2-Difunctionalised Compounds
- 24: Strategy XI: Radical Reactions in Synthesis
- 25: Two-Group Disconnections V: 1,4-Difunctionalised Compounds
- 26: Strategy XII: Reconnection
- 27: Two-Group C–C Disconnections VI: 1,6-diCarbonyl Compounds
- 28: General Strategy B: Strategy of Carbonyl Disconnections
- 29: Strategy XIII: Introduction to Ring Synthesis: Saturated Heterocycles
- 30: Three-Membered Rings
- 31: Strategy XIV: Rearrangements in Synthesis
- 32: Four-Membered Rings: Photochemistry in Synthesis
- 33: Strategy XV: The Use of Ketenes in Synthesis
- 34: Five-Membered Rings
- 35: Strategy XVI: Pericyclic Reactions in Synthesis: Special Methods for Five-Membered Rings
- 36: Six-Membered Rings
- 37: General Strategy C: Strategy of Ring Synthesis
- 38: Strategy XVII: Stereoselectivity B
- 39: Aromatic Heterocycles
- 40: General Strategy D: Advanced Strategy
- Index