![]()
1 Epidemiology of Kidney Disease
![]()
CHAPTER 1
Epidemiology of Chronic Kidney Disease
William M. McClellan1 & Friedrich K. Port2
1 Emory University School of Medicine, Atlanta, USA
2 Arbor Research Collaborative for Health, Ann Arbor, USA
Introduction
End-stage renal disease (ESRD) is defined by the cessation of effective kidney function and the substitution of renal replacement therapy (RRT), such as hemodialysis, peritoneal dialysis, or kidney transplantation, for native kidney function to sustain life. During the last 3 decades, an epidemic of ESRD has occurred in both industrialized and developing countries [1,2]. The epidemic increase in ESRD was initially attributed to the dissemination and adoption of RRT with the attendant extension of productive life. Although there is evidence that the rate of increase in ESRD incidence has abated in the USA, continuing increases in ESRD incidence rates after access to RRT becomes available to an entire population of a particular country have been documented by registries throughout the world [3].
The public health impact of the epidemic of ESRD is substantial. In the USA, it is estimated that the lifetime risk of being treated for ESRD is 2.5% for white men, 1.8% for white women, 7.3% for blackmen, and 7.8% for black women [4]. Life expectancy among individuals treated for ESRD is substantially shortened, and treatment is punctuated by frequent hospitalizations and progressive disability [3]. The economic costs of the epidemic are substantial as well, and the per-patient cost of care can exceed by severalfold the costs incurred by age-, gender-, and ethnicity-matched individuals in the general population. Furthermore, these costs only partially capture the full economic burden of ESRD, which includes the costs of chronic disability, premature mortality, and diminished quality of life.
Given the population cost burden of this epidemic of ESRD, it is increasingly recognized that strategies must be designed to increase the early detection and care of the antecedent diseases that contribute to this epidemic of end-organ failure [5,6]. There are multiple causes of kidney injury that result in ESRD, and the evidence-based diagnosis and management of these conditions are discussed in detail in subsequent chapters of this textbook. Common to each, however, is a continuum of progressive decline in kidney function that leads to a syndrome of chronic kidney disease (CKD), which is characterized by hypertension, anemia, renal/metabolic bone disease, nutritional impairment, neuropathy, impaired quality of life, and reduced life expectancy and which culminates in ESRD. The purpose of this chapter is to describe the definition of CKD and the measurement of the population-based health burden of CKD across the continuum of disease, from mild impairment to ESRD, as an essential foundation for the evidence-based management of kidney disease. Problems inherent in using biomarkers and prediction equations to define kidney function and detect CKD are discussed in chapter 2. The epidemiology of CKD is discussed in chapter 4, and risk factors associated with progressive loss of kidney function can be found in chapter 3. Chapter 2 examines how surveillance systems have been used to measure and improve the care of patients receiving RRT.
Definition of chronic kidney disease
CKD can be defined as the persistence for 3 or more months of structural and/or functional abnormalities of the kidney [7]. This definition replaces previous case definitions that described variable degrees of impaired kidney function [8,9]. The rationale for adopting a uniform case definition of CKD includes the need for 1) improved comparability across observational and clinical studies, 2) an improved capability for uniform comparisons of kidney disease incidence and prevalence, and 3) improved communications about diagnosis and treatment of kidney disease. The most important anticipated benefit of a common terminology is more effective communication with patients and the public.
The “structural” abnormalities used to define CKD are 1) microalbuminuria or overt proteinuria; 2) an abnormal urinary sediment as evidenced by the presence of red blood cells (RBCs), RBC casts, white blood cells (WBCs), WBC casts, tubular cells, cellular casts, granular casts, oval fat bodies, fatty casts, or free fat; and 3) abnormal findings on imaging tests, including ultrasound, intravenous pyelogram, computer tomography, magnetic resonance imaging, and nuclear scans. Overt proteinuria is defined as an increased urinary concentration of albumin and other proteins detected by routine laboratory measures (e.g. urine dipstick test for protein), and microalbuminuria is an increased albumin excretion that can be detected only by laboratory methods more sensitive than the standard protein assay that uses the urine dipstick.
The functional component of the definition of CKD uses creatinine-based estimates of clearance derived from the Modification of Diet in Renal Disease (MDRD) glomerular filtration rate (GFR) estimating equation or the Cockcroft–Gault creatinine clearance equation [10]. The derivation and use of these multivariate prediction equations are discussed in chapter 5. At present, no single method of GFR estimation is strongly recommended. Clinicians should choose a method that is appropriate for their population to determine the estimated GFR (eGFR) and assign a stage of kidney disease, always cognizant that failing to account for the modification of the complex association between serum creatinine and GFR by age, gender, and race is likely to lead to misclassification of kidney function and attendant errors in clinical decision making.
The available estimating equations are imprecise at higher levels of GFR, and there is great interest in revising them or identifying better filtration markers that will improve our ability to measure kidney function across the continuum of kidney performance from normal to ESRD [10]. The inherent imprecision of all methods of estimating GFR led to the decision to rank the degree of impaired kidney function into more global stages (levels) by the eGFR in the following manner:
Stage 1: eGFR > 90mL/min/1.73 m2 (with structural abnormalities)
Stage 2: 60–90 mL/min/1.73 m2 (with structural abnormalities)
Stage 3: 30–59 mL/min/1.73 m2
Stage 4: 15–29 mL/min/1.73 m2
Stage 5: <15 mL/min/1.73 m2
In addition to these eGFR ranges, the persistence of structural abnormalities for at least 3 months is necessary to assigning CKD stages 1 and 2, and stages 3–5 of CKD are defined by persistent impairments for greater than 3 months in the eGFR alone.
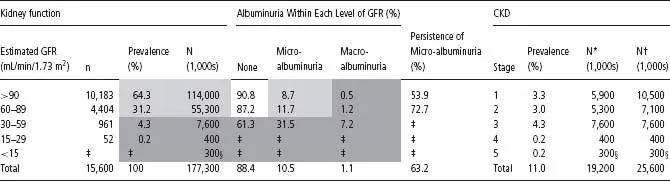
This staging algorithm is illustrated by using data from the US population aged 20 years and older (Table 1.1). The prevalence of CKD based on eGFR and presence and degree of proteinuria CKD is estimated to be 11% of the US population [7]. Over 50% of the prevalent disease is due to the presence of proteinuria among individuals with stage 1 (3.3%) and stage 2 (3.0%) CKD, and this proteinuria is largely due to microalbuminuria. Among individuals with stages 3–5 CKD, which are defined by eGFR alone, 85% of individuals have stage 3 disease (4.3%).
Kidney disease: improving global outcomes
The definition of CKD was reviewed at the 2004 “Kidney Disease: Improving Global Outcomes (KDIGO)” Controversies Conference [11]. Two further modifications were proposed to better adapt the staging algorithm for international use: 1) clinical judgment should be used to decide the relevance of nonproteinuric markers of kidney damage prior to diagnosing CKD in individuals without either proteinuria or reduced GFR; 2) individuals with a transplanted kidney should be considered as having CKD irrespective of other structural or functional markers. The KDIGO modified the CKD risk stratification by adding the letter T to denote CKD in a transplanted kidney and recommended that stage 5 CKD be modified by the letter D to denote RRT by dialysis [11].
International Classification of Diseases and kidney diseases
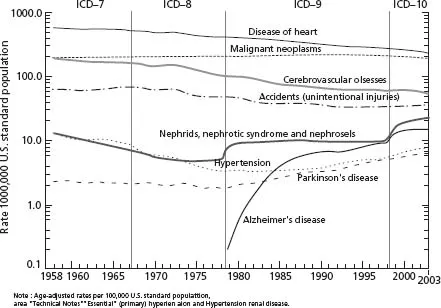
The International Classification of Diseases (ICD) classifies each condition that has given rise to the chain of events leading to death (underlying cause of death) as recorded on death certificates. The ICD is used by national vital statistics registries. At present, it provides the only uniform population-based case definition for international comparisons of the burden of disease attributable to earlier stages of CKD and, as such, is an important actuarial tool in defining the health burden of CKD across populations and, with certain limitations described below, temporally. The Ninth Revision of the ICD (ICD-9), used between January 1, 1979 and December 31, 1998, was replaced by ICD-10 on January 1,1999 [12].
Revisions of the ICD reflect the evolution of disease classification and emergence of new diseases, and they resolve administrative issues that have stemmed from a particular version of the codes. Clinicians should be aware that ICD revisions often introduce changes in the classification of an underlying cause of death. Comparisons of death rates due to specific causes, such as kidney disease, across different ICD revisions can be facilitated by using comparability ratios that relate rates from different time periods. The comparability ratio relating rate computed from ICD-9 (ICD-9 codes 580–589) and ICD-10 (ICD-10 codes N00–N07, N17–N19, and N25–N27) data is estimated to be 1.23, indicating that the new ICD-10 coding will result in a 23% increase in classification of deaths due to kidney disease compared with the ICD-9 codes [12]. This version-to-version difference is due, in part, to a change in the classification of ESRD from an unspecified disorder of the kidney in ICD-9 to ESRD (N18.0), ...