![]()
Chapter 1
Physical Extraction Operations
This chapter presents the unit operations of separation of the components of a mixture. These physical operations do not involve a chemical reaction. Only the bases of these processes are presented.
1.1. Solid-solid and solid-fluid separation operations
1.1.1. Flotation
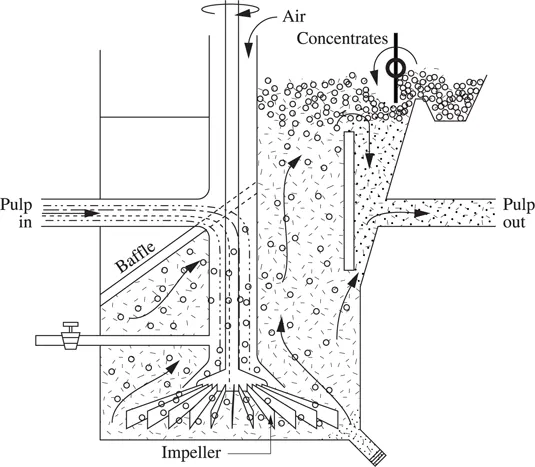
Flotation (“froth flotation”) is one of the primary mineral processing operations that is a beneficiation process. Flotation is a sorting (separation) process of chemically different solid particles by using surfactants and wetting agents [FUE 07]. The principle of froth flotation is as follows: after grinding into a fine powder, the solid particles to be separated are mixed with water. A water slurry (called a pulp) is then made. This pulp is treated with reactants (surfactants) that, by being selectively absorbed at the surface of certain particles, make them hydrophobic (having a greater affinity for air than for water).
This pulp of hydrophobic and hydrophilic particles is placed in a flotation cell with impellers (see Figure 1.1.1) and air bubbles are blown through it. Air bubbles attach themselves to the hydrophobic particles and carry them to the surface if the result of the surface tension, mass and buoyancy forces is negative. They are floated out in a stable froth (concentrates) that is skimmed off and discharged of its burden.
Collectors are ions of organic molecules that belong to the family of alkyldithiocarbonates or xanthates and dithiophosphates [RAO 04].
Flotation is one of the processes used for concentrating (beneficiating) complex ores, such as sulfide and carbonate ores of nonferrous metals (lead, copper, zinc, nickel). Sulfide ores can always be concentrated by flotation with good yields. Flotation is the most commonly used technique for separating minerals from their gangue (silicates are wetted by water, sulfides are non-wetted). The ore has to be subjected to a grinding until particles with a size lower than 150 μm are obtained. This operation has a major economical importance (units have a treatment capacity of more than 100,000 tons of ore per day).
Flotation also allows the separation of sulfides: in the treatment of zinc-lead sulfide ores, nickel-copper sulfide concentrates and nickel-copper mattes (see Chapter 10, Figure 10.5.2) leading to a concentrate rich in one sulfide and a concentrate rich in the other.
In the refining operations of steel and aluminum, flotation is used to remove from the liquid phase oxide inclusions formed during the deoxidation of steel (see [VIG 11b], Chapter 7, section 7.2.3) or present in [MIR 09]. This operation is performed by injection of argon bubbles that attach themselves to the inclusions.
The removal of sulfur S°, which forms in the leaching of zinc sulfides, is performed by flotation (see [VIG 11b], Chapter 1, section 1.5.2.2).
1.1.2. Settling under gravity
Settling under gravity is the natural separation of solids that are suspended in a liquid. Settling can be accelerated by coagulation of the particles with flocculating agents.
1.1.3. Centrifugation
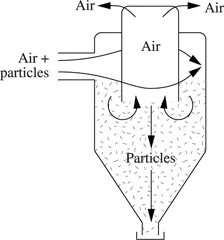
The gas-solid separation is carried out in cyclones (see Figure 1.1.2). The solid particles carried along by a gas flowing at a high rate, due to the centrifugal force, end up on the walls of the cyclone during a downward helical journey and fall to the lower conical part, whereas the gas escapes from the higher part.
For liquid-solid suspensions, the separation by centrifugation is obtained by rotating the suspension.
1.1.4. Filtration
Ordinary filtration is used in hydrometallurgical operations. For aqueous suspensions, the separation of the particles resulting from a precipitation is obtained by filtration, which is carried out by passing the slurry through a natural or synthetic cloth that will not permit passage of solids. The cloth should offer minimal resistance to water flow and act mainly as a support for the deposited solids that form the effective filter bed. The water may be forced through the filter under pressure or drawn through by suction.
The presence of non-metallic inclusions in cast iron, steels and aluminum alloys is very detrimental to product quality. These very fine oxide inclusions (of 1–3 μm) are formed when the steel is deoxidized with various deoxididizers, such as aluminum, silicon, etc. They do not separate into the slag phase during these refining operations in spite of their low density. Filtration of liquid metals prior to casting is used to remove inclusions (see [VIG 11b], Chapter 7, section 7.2.3).
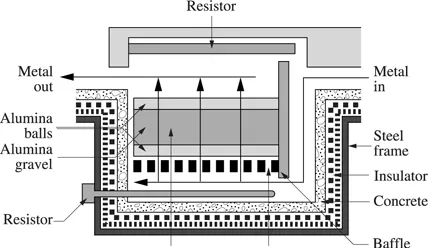
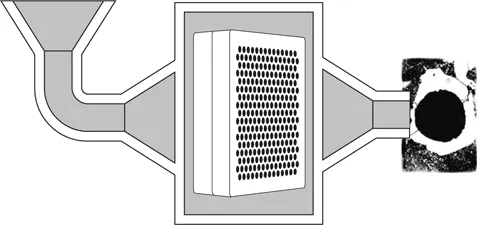
The ceramic (alumina) filters are made either of a bed of spherical particles or of a monolith bearing parallel cylindrical pores with a diameter of about 1 mm. The pore diameter in a deep bed filter can be 100 times as large as the filtered particle diameter. The particles are captured by adsorption on the pore walls throughout the entire depth of the filter. The flow rate of the liquid metal in these pores is thus a significant parameter for the efficiency of these filters. The ceramic (alumina) used allows the filtration of aluminum alloys (see Figure 1.1.3), copper alloys, cast iron (see Figure 1.1.4) and of super-alloys.
A filter, such as the one presented in Figure 1.1.3, with a height of 1.5 m and a global surface of 2.7 m2 can treat 25 tons of aluminum per hour. It contains 2.5 tons of liquid metal [BRE 95].
1.2. Separation operations of the components of a fluid phase
1.2.1. Condensation
Condensation allows separation of components of a gaseous phase by cooling. Condensation is carried out in zirconium and titanium metallurgy in order to separate the gaseous chlorides obtained by carbochlorination of the ores (see Chapter 10, Figure 10.10.1).
1.2.2. Vacuum distillation
In zirconium metallurgy, after the Kroll reduction that produces a pseudo Zr-Mg alloy, vacuum distillation producing a vaporization of magnesium allows the separation of magnesium and magnesium chloride from titanium or zirconium, leaving a sponge (see Chapter 10 Figure 10.10.1).
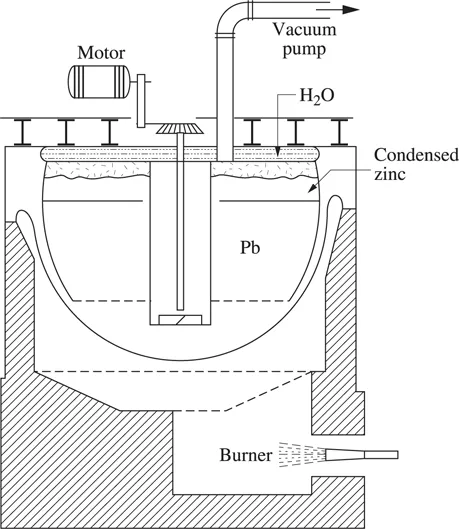
Lead bullion is refined (see Chapter 10 Figure 10.7.1) using a vacuum distillation operation, to remove zinc whose content reaches ≈0.55%. At 600°C, under a vacuum of 0.05 mbar, the volatilized metallic zinc condenses on the higher part of a tank cooled down by water (see Figure 1.2.1). A mechanical vacuum pump produces a residual pressure of about 0.05 mbar. A temperature of 600°C, a stirring time of five hours reduces the zinc content in lead from 0.50% to 0.05%.
1.2.3. Liquation
After cooling, a two-component liquid phase is separated into two liquid phases. This is liquation. One of the phases is rich in component A and the other is rich in component B when the phase diagram of the A-B binary solution presents a miscibility gap.
Liquation is also an operation in which impurities in a metal are removed by cooling the mixture to just above the melting point of the pure metal. Where the solubility of the impurity decreases and precipitation of this impurity or a compound occurs.
This operation is used in zinc metallurgy, in the pyrometallurgical processing route based on the Imperial smelting process, after zinc condensation by lead to separate lead from zinc (see Chapter 4, Figure 4.4.1 and Chapter 10 Figure 10.6.1). The zinc-lead equilibrium ...