![]()
Chapter 1
Introduction
1.1 Similarities Between Polymers and Nanotubes
The introduction of synthetic polymers at the beginning of the twentieth century led to a revolution in the way people live their everyday lives. Polymers are found in homes, offices, and places of business all over the world; in fact, one could likely find synthetic polymers in every home or apartment in the world. From a molecular viewpoint, polymers have been around much longer than humans; many types of biomolecules including DNA, RNA, and proteins are polymers. Industrially, natural rubber has been used for various applications since the middle of the nineteenth century with the key technological step being the vulcanization of rubber first reported by Charles Goodyear in 1839. However, when Hermann Staudinger first proposed the existence of long-chain molecules just after World War I, many scientists in the established scientific community discounted that such molecules could possibly exist. Of course, Staudinger was correct; polymers are molecules of very high molecular weight. The per annum growth rate of polymer production from the time of Staudinger to 1975 was 15%; since 1975 the rate has slowed down to 8%. Even at this lower rate, the production of polymers far outstrips the per annum increase in the number of people on the planet indicating that people are using more and more polymers. The amount of polymers synthesized each year is approximately 50 pounds per person on the planet, and is more than twice that for people living in Western Europe or the United States. The author has a hard time even imagining a world without synthetic polymers (not to mention the fact he might be out of a job!).
One hundred years from now it might be said that carbon nanotubes had a similar life cycle to polymers, except that the key dates were shifted by about 100 years. The “birth” of carbon nanotubes is generally ascribed to the seminal publication by Iijima in 1991.1 However carbon nanotubes have been around for a much longer time, just as polymers had been around long before Staudinger's hypothesis. A paper in Russian by L. V. Radushkevich and V. M. Lukyanovich 2 in 1952 first showed transmission electron microscopy images of carbon nanotubes. The first patent for something that, in hindsight, was clearly a carbon nanotube was issued to Hyperion Catalysis in 1987 (U.S. patent 4,663,230). Other examples available in the open literature prove the existence of carbon nanotubes long prior to 1991. In fact, Monthioux and Kuznetsov 3 provided a rather detailed answer to the question “Who should be given the credit for the discovery of carbon nanotubes?”. It has also been stated that the confusion around who discovered carbon nanotubes has prevented the awarding of any Nobel Prize for their discovery.4 Regardless, the explosion of interest in carbon nanotubes definitely dates from the Iijima's paper and, as Monthioux and Kuznetsov state, “the undoubted tremendous impact of the 1991 Iijima paper came from the right combination of favorable factors: a high quality paper, a top-rank journal read by all kinds of scientists, including those involved in basic research and fundamental physics, a boost received from its relation to the earlier worldwide research hit (fullerenes), and a fully mature scientific audience ready to surf on the ‘nano’ wave.”
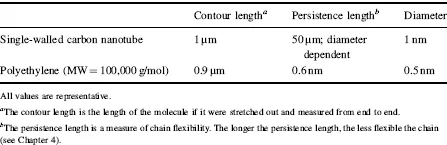
At the beginning of the twenty-first century, possible applications of nanotubes are being explored in medicine, electronics, energy, and polymers. Similar to polymers, nanotubes are attractive for a number of applications because of their unique architecture; nanotubes are just about the only material that has a diameter on the order of 1 nm, lengths on the order of microns, and are rigid. The fact that nanotubes are hollow is another unique property of nanotubes. Beyond the parallels in their history, carbon nanotubes and polymers are very much alike on a molecular basis as well. Consider Table 1.1 that lists the properties of an individual chain of the most common polymer, polyethylene, at a typical commercial length and an individual single-walled carbon nanotube at a typical commercial length.
Table 1.1 Comparison Between Polymers and Carbon Nanotubes.
The only significant difference in the values shown in Table 1.1 is for the persistence length. However, this stiffness difference means a great deal with respect to properties. As anyone who has worked with a carbon nanotube mat could tell you, a difference in chain stiffness means that the properties of a bulk polymer sample and a bulk nanotube sample are very different. Because of their flexibility, polymers can be melted and molded like other liquids; nanotubes cannot be melted and thus never form a liquid phase. Both nanotubes and polymers can be dispersed in liquids; however, upon drying a polymer can form a dense, nonporous film while nanotubes form porous films. There are other significant differences between the two materials not related to chain stiffness; for example, most polymers are thermal and electrical insulators, while nanotubes are thermal and electrical conductors. Nanotube electrical, mechanical, and thermal properties, as well as others, will be presented in Chapter 2.
In addition to history and size, a third important parallel between polymers and carbon nanotubes is the methods by which these two materials are synthesized. One type of very commercially important polymer, including polyethylene, is made using a solid catalytic particle and a gas that contains the reactive ingredient. The most commercially important synthetic method for carbon nanotubes also involves a solid catalytic particle and a gas that contains the reactive ingredient. One of the most important properties of polymers is the small per pound cost; carbon nanotubes definitely do not have this characteristic at present! The most significant component of the cost of a polymer is the cost of the reactive ingredient(s), that is, the monomer. In carbon nanotubes, the reactive ingredient cost is essentially a negligible portion of the total cost. In fact, the most common monomer used for carbon nanotubes, carbon monoxide, is actually less expensive than the most inexpensive polymer monomers. This difference in cost is due to one significant difference between the synthesis of polymers and that of carbon nanotubes. The yield, that is, pounds of product per pounds of catalyst, is on the order of 10,000,000 : 1 for polymers and at best 50 : 1 for carbon nanotubes. Not only is the catalyst cost substantial, but the carbon nanotube product must also be purified in order to remove the catalyst. In polymers, the catalyst is such a small part of the material that usually no purification step to remove catalyst is performed. Other methods can be used to make carbon nanotubes, but these methods are even more expensive and unsuitable for translation into processes of the size necessary for most commercial applications. Various synthetic methods to produce carbon nanotubes will be discussed in Chapter 2.
Synthetic polymers, with some notable exceptions such as silicone breast implants, certain plasticizers in poly(vinyl chloride), and bisphenol A in polycarbonate, are considered to have little or no effect on human health, and in fact have been used extensively in biomedical devices to improve human health. Nanotubes, because of their small size, will easily become airborne if special precautions are not taken. Since the effect of airborne nanotubes on human health is not understood (this issue is beyond the scope of this book), nanotubes must be handled carefully. This characteristic has a significant effect in the way in which nanotubes are processed when being combined with polymers, with the practical effect that the manufacturer of the tubes often must sell a product that is not simply nanotube powder.
1.2 Organization of the Book
Polymers are typically categorized according to repeat unit structure. However, with a given repeat unit structure, polymers can be categorized with respect to chain architecture, that is, as linear, branched, and so on. The six-membered planar graphene ring serves as the repeat unit for all carbon nanotubes and all carbon nanotubes can be thought of as sheets of repeating graphene rings that have been rolled up into cylinders. Even so, carbon nanotubes can also be categorized structurally. Carbon nanotubes can be separated into one of the three types: multi-walled carbon nanotubes (MWCNTs), which consist of 3–30 concentric cylinders having an outside diameter generally from 5 to 20 nm; single-walled carbon nanotubes (SWCNTs), which consist of single cylinders having a diameter from 0.7 to 1.5 nm; and double-walled carbon nanotubes (DWCNTs), which consist of two concentric cylinders. The way in which the graphene sheets are rolled can also be different and this difference has a significant impact on properties. Rolling will be described more completely in Chapter 2; differences in rolling mean that over 50 types of nanotubes exist in many commercial SWCNTs with a much larger number in commercial MWCNTs. Like polymers, there is a broad distribution of tube lengths in a given sample. Hence, a single sample of carbon nanotubes is an extremely complicated mixture of many different products. A further complication rests in the fact that the efficacy of purification procedures to remove catalyst and reaction by-products can be very different. Hence, nominally identical materials from two different manufacturers are guaranteed to be different. A given manufacturer must also be very careful to ensure that batch-to-batch variations in nanotubes are not significant as well.
A number of companies are producing ton-size quantities of nanotubes for the polymer market. Predicting exactly which manufacturers will be making nanotubes at this scale or, for that matter, at a research scale at the time when the book is read would be an exercise in fortune telling. The interested reader can consult the online encyclopedia Wikipedia (http://en.wikipedia.org/wiki/Carbon_nanotube#List_of_Carbon_Nanotube_Suppliers) for an up-to-date complete (or almost complete) list of companies currently supplying nanotubes. Sending the reader to Wikipedia I don't believe is an exercise in fortune telling; Wikipedia will be around much longer than some of the companies currently listed as being manufacturers of carbon nanotubes!
Normally, single-walled tubes in particular are in an aggregated state in which the long axes of tubes are aligned in the same direction and the minimum distance between the tubes is roughly equal to the distance between the concentric cylinders in MWCNTs. This aggregated morphology is termed bundles. MWCNTs may also be bundled, but both MWCNTs and SWCNTs are also often found aggregated on a larger scale like a ball of yarn or string. Most applications, including those that involve polymers, would ideally use individually dispersed nanotubes. To reach this goal two issues must be addressed: deaggregation of nanotubes and the prevention of reaggregation during subsequent processing steps. As will become clear in Chapter 3, only perhaps in samples made by the most careful research laboratories where extraordinary efforts are used might there be polymers containing only individually dispersed tubes. So not only is it difficult to compare results between laboratories because different tubes are used, even in the case where the same tubes are used the dispersion is likely to be different and hence results are still difficult to compare.
Once the existence of carbon nanotubes was recognized by the scientific community, polymer composites were one of the most obvious applications. Carbon fibers, which have diameters 100–1000 times larger than nanotubes and are not hollow, have been used as fillers in polymer composites for over 40 years. Hence, the intellectual jump to use carbon nanotubes in composites was an easy one to make. A large number of companies are currently manufacturing products that contain carbon nanotubes in polymers or are considering products that contain carbon nanotubes in polymers. Partly because of health concerns, the production model for nanotubes in thermoplastics will likely be for a carbon nanotube manufacturer or partner to mix nanotubes with a given polymer at a much higher percentage than what will be used in the final product, i.e., make a masterbatch. Most economical processes for mixing nanotubes with thermoplastics involve high-shear mixing in a twin-screw extruder or the equivalent. The end user will then take the masterbatch and dilute it with polymer resin in order to make the final product. Some high-end uses will likely disperse nanotubes in a solvent to facilitate mixing. For thermosets with randomly organized nanotubes, it is likely that the resin will be sold with already dispersed nanotubes, and the final end user will perhaps dilute the nanotubes and then cure the product into its desired shape. As will become clear in Chapter 3, carbon nanotubes can be fashioned into yarns or fibers ideal for continuous composites. Even in this case, health concerns will likely force companies to sell prepreg, that is, a partially cured sheet of thermoset resin that contains nanotube fibers.
As stated in the previous paragraph, there are two broad classes of polymers: thermoplastics and thermosets. The difference between a thermoplastic and a thermoset is that the former consists of isolated chains (think of a ball of string that is cut into many pieces and the pieces are mixed together) while the latter is made up of interconnected chains (think of a net, although the tie points are not as regular as a net). Most, thermoplastics can be melted, that is, form a high-viscosity liquid, at elevated temperatures. Thermosets cannot be melted at any temperature without chemical degradation. Nanotubes are important for both kinds of polymers.
Polymer matrix nanocomposites, that is, mixtures of polymer and filler with the latter having at least one nanoscale dimension, have a number of interesting phenomena related to the fact that a large fraction of polymer is close to a solid interface. Although it has been understood for a great many years that interfaces, such as the amorphous–crystalline interface in a semicrystalline polymer, can affect the properties of polymers significantly, the proliferation of nanocomposites has popularized the fact that polymer properties near an interface are very different. Coupling that characteristic with high aspect ratio nanotubes means that phenomena are seen in nanotube composites that are seen nowhere else. One striking example is the unique polymer crystalline morphologies that can be achieved with carbon nanotubes. Chapter 4 describes how nanotubes affect and alter polymer physics, and these alterations contribute to some of the unique properties of carbon nanotube–polymer composites.
From a simple rule of mixtures, as well as more complicated models that will be described in Chapter 5, the promise of carbon nanotubes in making ultrastrong polymer composites is clear. At a nanotube volume percent of 10%, a common, inexpensive polymer such as polyethylene or polypropylene could be transformed into a polymer that has a stiffness and strength equivalent to a high-performance polymer such as Kevlar™. Alternatively, 10% nanotubes could be added to Kevlar™ or some other very high-modulus/high-strength polymer, to create a product having no counterpart in specific strength or stiffness. As will become clear, no such improvements have been seen at high loading levels. Carbon nanotubes added to polymers have led to marginal improvements in strength and stiffness, and the resulting composites have followed mixing rules to about 1% nanotube content; however, improvements in mechanical performance have not been large enough to use nanotubes instead of other less costly fillers. Some commercial success with respect to mechanical properties has been found by either replacing part of another filler or adding nanotubes as an additive to an already filled composite. Nanotubes that have been drawn into fibers or yarns and then incorporated into polymers can be loaded to significantly higher fractions with good enhancements in mechanical properties. The market is significantly smaller than the case where the nanotubes are mixed together with a polymer melt, but producing nanotube fibers and then adding a low-viscosity resin that is then cured holds some promise.
A property of which there is no question that the use of nanotubes is being driven by increases in performance is electrical conductivity. Because ...