
eBook - ePub
Handbook of Biodegradable Polymers
Isolation, Synthesis, Characterization and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Biodegradable Polymers
Isolation, Synthesis, Characterization and Applications
About this book
A comprehensive overview of biodegradable polymers, covering everything from synthesis, characterization, and degradation mechanisms while also introducing useful applications, such as drug delivery systems and biomaterial-based regenerative therapies. An introductory section deals with such fundamentals as basic chemical reactions during degradation, the complexity of biological environments and experimental methods for monitoring degradation processes.
The result is a reliable reference source for those wanting to learn more about this important class of polymer materials, as well as scientists in the field seeking a deeper insight.
The result is a reliable reference source for those wanting to learn more about this important class of polymer materials, as well as scientists in the field seeking a deeper insight.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Handbook of Biodegradable Polymers by Andreas Lendlein,Adam Sisson in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.
Information
Edition
11
Polyesters
1.1 Historical Background
1.1.1 Biomedical Applications
Biomaterials are defined as any materials intended to interface with biological systems to analyze, treat, or replace any tissue, organ, or function of the body [1]. The current trend in biomaterial development is shifted toward the use of biodegradable materials that have definite advantages in the fields of tissue engineering [2] and drug delivery [3]. The general principle is to use a material that achieves a specific therapeutic task and is subsequently, over time, degraded and removed harmlessly from the body. As an increasingly relevant part of the medical device and controlled release industry, biodegradable polymers are used to fabricate temporary scaffolds for tissue regeneration, medical sutures, and nano- or microscale drug delivery vehicles [4–6].
The important properties that are required for biodegradable biomaterials can be summarized as follows:
- Nontoxic and endotoxin-free, aiming to minimalize unwanted foreign body responses upon implantation.
- Degradation time should be matched to the regeneration or required therapy time.
- Mechanical properties must be suited to the required task.
- Degradation products should be nontoxic and readily cleared from the body.
- Material must be easily processed to allow tailoring for the required task.
Although natural polymers such as collagen have been used in medical applications throughout history, synthetic polymers are valuable also, as they allow us to tailor properties such as mechanical strength and erosion behavior. Naturally occurring biopolymers are typically degraded by enzymatic means at a rate that may be difficult to predict clinically. Furthermore, natural polymers may have unwanted side effects arising from inherent biological activity. This has led to the widespread use of biodegradable synthetic polymers in therapeutic applications. Of this class, biodegradable aliphatic polyesters, which are degraded hydrolytically, are by far the most employed.
1.1.2 Poly(Hydroxycarboxylic Acids)
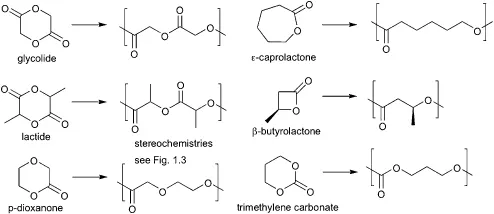
All polyesters are, in principle, hydrolytically degradable. However, only (co)polyesters with short aliphatic chains between ester bonds typically degrade over the time frame required for biomedical applications. The major group of this material are the poly(hydroxycarboxylic acids), which are prepared via ring-opening polymerization of lactones or cyclic diesters. Indeed, the first biodegradable polyester used as a medical suture in the 1960s was based on the polyglycolide. Scheme 1.1 shows the most common monomers and the polymers they produce. These can be summarized as diglycolide, stereogenic dilactides, lactones such as ε-caprolactone and stereogenic β-butyrolactone, the cyclic trimethylene carbonate, and p-dioxanone. As the polymerization methods of these monomers are broadly applicable to each, copolymers such as poly(lactide-co-glycolide) are readily produced.
Scheme 1.1 Common cyclic monomers for the preparation of polyester derivatives.
Another source of poly(hydroxycarboxylic acids) is from bacteria, which store polyesters as their energy source [7]. These polymers are known as polyhydroxyalkanoates (PHAs) in the literature. The most common polymer derived from bacteria is poly(3-hydroxybutyrate), which has the same structure as the polymer which can be obtained from optically active β-butyrolactone [8]. Poly(3-hydroxybutyrate) formed in this way is strictly stereoregular, showing the (R) configuration. Biotechnologically produced polymers are discussed in more details in Chapter 2 of this handbook.
1.2 Preparative Methods
1.2.1 Poly(Hydroxycarboxylic Acid) Syntheses
Polyesters can be synthesized via the direct condensation of alcohols and acids. This may take the form of condensing dialcohols and diacids, for example, AA + BB systems, or the direct condensation of hydroxycaboxylic acid monomers, for example, AB systems. Various catalysts and coupling reagents may be used but typically the polyesters formed in this manner have low and uncontrolled molecular weight and are not suitable for biomedical applications. The majority of cases where a high degree of polymerization was obtained came via ring-opening polymerizations of cyclic monomers of the type shown in Scheme 1.1 [9]. The cyclic dilactones are prepared from the corresponding hydroxycarboxylic acid by elimination of water in the presence of antimony catalysts such as Sb2O3 [10]. These dimers have to be purified rigorously if high degrees of polymerization are sought, as impurities such as water and residual hydroxycarboxylic acids can hinder polymerization. Enantiomerically pure lactic acids are typically produced by fermentation.
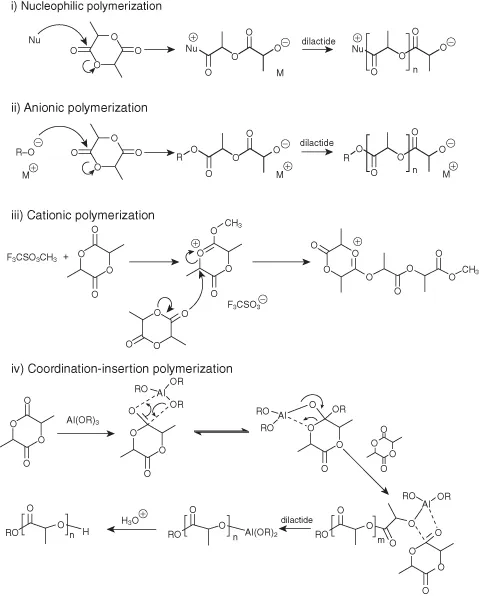
Ring-opening polymerizations may be initiated by nucleophiles, anionically, cationically, or in the presence of coordinative catalysts. Representative mechanisms are shown in Scheme 1.2. However, precise mechanisms may vary from case to case and are an ongoing important area of study [11, 12]. As a testament to the popularity of the ring-opening polymerization approach, over 100 catalysts were identified for the preparation of polylactide [13].
Scheme 1.2 Overview of various mechanisms relevant to polylactide synthesis.
The typical complex used for the industrial preparation of polyglycolide derivatives is tin(II)-bis-(2-ethylhexanoate), also termed tin(II)octanoate. It is commercially available, easy to handle, and soluble in common organic solvents and in melt monomers. High molecular weight polymers up to 106 Da and with narrow polydispersities are obtained in a few hours in bulk at 140–220 °C. Approximately 0.02–0.05 wt% of catalyst is required. Care must be taken when polymerizing dilactides, if stereochemistry is to be preserved. This means that milder conditions are to be selected relative to the homopolymerization of diglycolide.
For the copolymerization of dilactide and diglycolide catalyzed with tin(II)octoate, different reactivities are observed. A chain with a growing glycolide end will add a further diglycolide with a preference of 3:1. With a terminal lactide unit, the preference for diglycolide is 5:1. Due to this, glycolide blocks tend to form, separated by single dilactides. One possibility to improve the homogeneity of the composition of the obtained polyesters is the online control of the monomer ratio by addition of further monomer. However, this method is technically complicated.
The mechanism is a nonionic coordinated insertion mechanism, which is less prone to the side reactions commonly found in ionic polymerizations, such as transesterification or racemization [14, 15]. It has been found that the addition of alcohols to the reaction mixture increases the efficiency of the tin catalyst albeit by a disputed mechanism [16]. Although tin(II)octoate has been accepted as a food additive by the U.S. FDA, there are still concerns of using tin catalysts in biomedical applications.
Aluminum alkoxides have been investigated as replacement catalysts. The most commonly used is aluminum isopropoxide, which has been largely used for mechanistic studies [17]. However, these are significantly less active than tin catalysts requiring prolonged reaction times (several hours to days) and affording polymers with molecular weights generally below 105 Da. There are also suspected links between aluminum ions and Alzheimer’s disease. Zinc complexes, especially zinc(II)lactate, are a plausible replacement with low toxicity and activities of the same order as aluminum complexes [18]. Zinc powder may also be used but then the active species has been identified as zinc(II)lactate in the preparations of polylactide [19]. Iron salts and particularly iron(II)lactate show comparable activity, but prolonged reaction times mean that some racemization occurs in the synthesis of high molecular weight (50,000 Da) poly(L-lactide) [20].
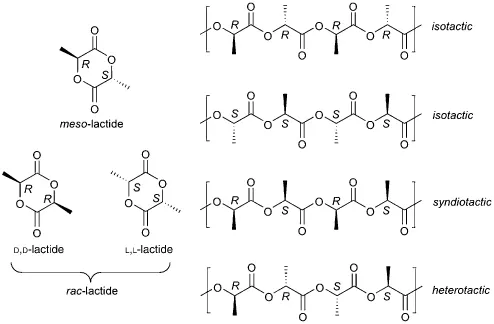
As can be seen in Scheme 1.3, polylactides can exist in isotactic, syndiotactic, and heterotactic blocks. The configuration has obvious consequences for the material properties of the final polymer. While the ring-opening polymerization of L,L-dilactide or D,D-dilactide leads to isotactic polymers, the polymers of the rac-dilactide should consist mainly of isotactic diads. This is due to the fact that rac-dilactide is commonly used as a mixture of D,D- and L,L-dilactide with very little meso-dilactide content. The formation of syndiotactic diads is expected in the case of meso-dilactide polymerization, but the longer range sequence structure of such polymers is typically atactic. Due to the expense of producing stereopure dilactides, a kinetic resolution procedure was developed whereby chiral SALEN (salicylimine) ligands in combination with aluminum isopropoxide catalyst produced isotactic polylactides from rac-dilactide. Optical purities were high at 50% conversion; kinetics show that the catalyst system has a 28:1 preference toward one isomer. By choosing the appropriate SALEN ligand enantiomer, selective polymerization of either L,L- or D,D-dilactide could be achieved [21]. Similar approaches using SALEN ligands have been employed to produce syndiotactic and heterotactic polylactides [22].
Scheme 1.3 Stereochemical possibilities observed with polylactide synthesis.
Tin(II)octoate is also the most common catalyst used for the polymerization of cyclic lactone monomers such as ε-caprolactone; although the mechanism of polymerization may differ [23]. In addition, rare-earth metal complexes have been shown to work as effective catalysts leading to high molecular weight polylactones with low polydispersity [24, 25]. An efficient cationic ring-opening polymerization of lactones has been developed using scandium trifluoromethanesulfonate as catalyst. Poly(ε-caprolactone) with narrow polydispersity and a molecular weight in the order of 104 Da was produced in quantitative yield after 33 h at room temperature in toluene. Only 0.16 mol% of catalyst was required. Similar results were obtained for poly(δ-valerolactone). Notably, the reaction was relatively tolerant to the presence of moisture and other contaminants [26].
1.2.2 Metal-Free Synthetic Processes
The use of low molecular weight organic molecules to ca...
Table of contents
- Cover
- Series page
- Title page
- Copyright page
- Preface
- List of Contributors
- 1 Polyesters
- 2 Biotechnologically Produced Biodegradable Polyesters
- 3 Polyanhydrides
- 4 Poly(Ortho Esters)
- 5 Biodegradable Polymers Composed of Naturally Occurring α-Amino Acids
- 6 Biodegradable Polyurethanes and Poly(ester amide)s
- 7 Carbohydrates
- 8 Biodegradable Shape-Memory Polymers
- 9 Biodegradable Elastic Hydrogels for Tissue Expander Application
- 10 Biodegradable Dendrimers and Dendritic Polymers
- 11 Analytical Methods for Monitoring Biodegradation Processes of Environmentally Degradable Polymers
- 12 Modeling and Simulation of Microbial Depolymerization Processes of Xenobiotic Polymers
- 13 Regenerative Medicine: Reconstruction of Tracheal and Pharyngeal Mucosal Defects in Head and Neck Surgery
- 14 Biodegradable Polymers as Scaffolds for Tissue Engineering
- 15 Drug Delivery Systems
- 16 Oxo-biodegradable Polymers: Present Status and Future Perspectives
- Index