
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Medical Biochemistry at a Glance
About this book
Offering a concise, illustrated summary of biochemistry and its relevance to clinical medicine, Medical Biochemistry at a Glance is intended for students of medicine and the biomedical sciences such as nutrition, biochemistry, sports science, medical laboratory sciences, physiotherapy, pharmacy, physiology, pharmacology, genetics and veterinary science. It also provides a succinct review and reference for medical practitioners and biomedical scientists who need to quickly refresh their knowledge of medical biochemistry.
The book is designed as a revision guide for students preparing for examinations and contains topics that have been identified as 'high-yield' facts for the United States Medical Licensing Examination (USMLE), Step 1.
This third edition:
- Has been thoroughly revised and updated and is now in full colour throughout
- Is written by the author of the hugely successful Metabolism at a Glance (ISBN 9781405107167)
- Features updated and improved clinical correlates
- Expands its coverage with a new section on Molecular Biology
- Includes a brand new companion website of self-assessment questions and answers at www.ataglanceseries.com/medicalbiochemistry
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part 1: Acids, bases and pH
1
Acids, bases and hydrogen ions (protons)
Definition of PH
pH is defined as “the negative logarithm to the base 10 of the hydrogen ion concentration”,
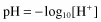
For example, at pH 7.0, the hydrogen ion concentration is 0.000 000 1 mmoles/litre or 10−7 mmol/l.
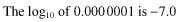
Therefore, the negative log10 is −(−7.0), i.e. +7.0 and hence the pH is 7.0.
What Is PH?
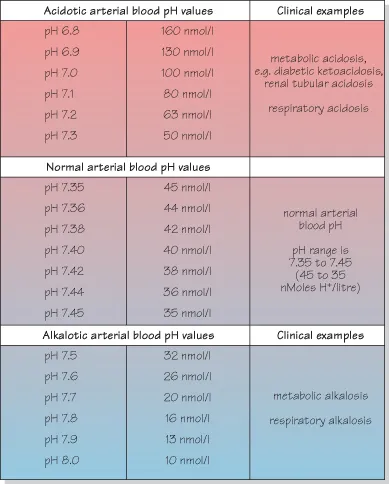
pH is “the “power of hydrogen”. It represents “the negative logarithm10 of the hydrogen ion concentration”. So why make things so complicated: why not use the plain and simple “hydrogen ion concentration”? Well, the concept was invented by a chemist for chemists and has advantages in chemistry laboratories. In clinical practice we are concerned with arterial values between pH 6.9 and 7.9. However, chemists need to span the entire range of pH values from pH 1 to pH 14. Values in terms of pH enable a convenient compression of numbers compared with the alternative which would be extremely wide-ranging as shown in Fig. 1.3. Figure 1.6 shows the normal reference range for pH in blood and, in extremis, fatal ranges that may be seen in acidotic or alkalotic diseases.
Figure 1.1 Revision of logarithms.
Access a high quality version of this image at http://booksupport.wiley.com.
Access a high quality version of this image at http://booksupport.wiley.com.
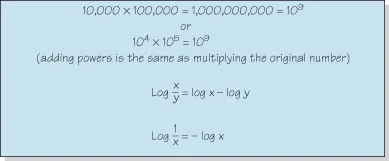
Figure 1.2 Examples of numbers and their logarithms.
Access a high quality version of this image at http://booksupport.wiley.com.
Access a high quality version of this image at http://booksupport.wiley.com.
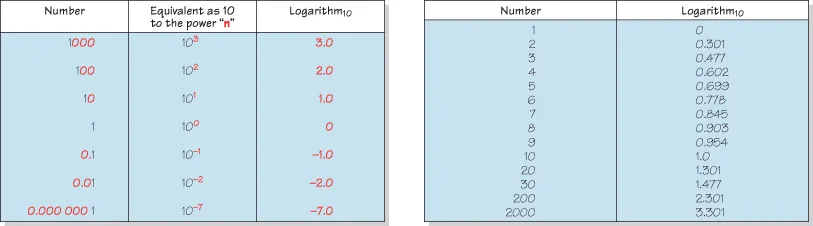
Figure 1.3 Understanding units.
Access a high quality version of this image at http://booksupport.wiley.com.
Access a high quality version of this image at http://booksupport.wiley.com.
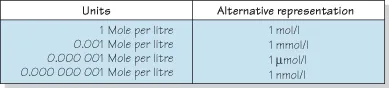
Figure 1.4 Brønsted and Lowry definition of acids and bases.
Access a high quality version of this image at http://booksupport.wiley.com.
Access a high quality version of this image at http://booksupport.wiley.com.
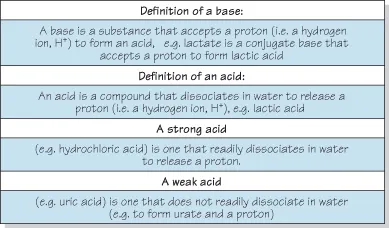
Figure 1.5 pH and equivalent values.
Access a high quality version of this image at http://booksupport.wiley.com.
Access a high quality version of this image at http://booksupport.wiley.com.
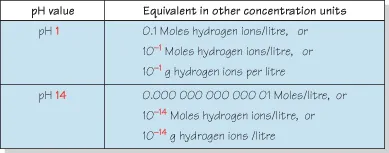
Figure 1.6 Examples of pH values seen in clinical practice.
Access a high quality version of this image at http://booksupport.wiley.com.
Access a high quality version of this image at http://booksupport.wiley.com.
The PH Scale Is Not Linear
”The patient’s blood pH has changed by 0.3 pH unit” means it has doubled (or halved) in value.
It is sometimes stated that “the patient’s arterial blood pH has increased/decreased by, for example, 0.2 pH unit”. However, notice that because of the logarithmic scale, this can misrepresent the true change in traditional concentration units. For example, a fall of 0.2 pH units from pH 7.20 to pH 7.00 represents 37 nmol/l, whereas a decrease from pH 7.00 to pH 6.8 represents a change of 60 nmol/l.
Also note that because the log10 of 2 = 0.3 (that is 2 = 100.3), a decrease in pH by 0.3, e.g. from pH 7.40 to pH 7.10, represents a two-fold increase in H+ concentration, i.e. from 40 nmol/l to 80 nmol/l. Similarly, an increase in pH from pH 7.40 to pH 7.70 represents a fall in H+ concentration from 40 nmol/l to 20 nmol/l.
The Henderson–Hasselbalch Equation
A weak acid dissociates as shown:
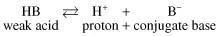
where HB is the weak acid that dissociates to a proton H+ and its conjugate base B−. NB Traditionally authors refer to the conjugate base as “A−”, i.e. the initial letter of acid, which is perhaps confusing.
Therefo...
Table of contents
- Cover
- Dedication
- Title page
- Copyright page
- Preface to the third edition
- Acknowledgements to the third edition
- Figure key
- SI/mass unit conversions
- Part 1: Acids, bases and pH
- Part 2: Structure of amino acids and proteins
- Part 3: Formation of ATP: oxidation and reduction reactions
- Part 4: Carbohydrates
- Part 5: Enzymes and regulation of pathways
- Part 6: Lipids and lipid metabolism
- Part 7: Metabolism of amino acids and porphyrins
- Part 8: Vitamins
- Part 9: Molecular biology
- Part 10: Diagnostic clinical chemistry
- Index
- Artwork
- Wiley End User License Agreement
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Medical Biochemistry at a Glance by J. G. Salway in PDF and/or ePUB format, as well as other popular books in Medicine & Medical Theory, Practice & Reference. We have over one million books available in our catalogue for you to explore.