
eBook - ePub
Introduction to Pharmaceutical Chemical Analysis
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Introduction to Pharmaceutical Chemical Analysis
About this book
This textbook is the first to present a systematic introduction to chemical analysis of pharmaceutical raw materials, finished pharmaceutical products, and of drugs in biological fluids, which are carried out in pharmaceutical laboratories worldwide.
In addition, this textbook teaches the fundamentals of all the major analytical techniques used in the pharmaceutical laboratory, and teaches the international pharmacopoeias and guidelines of importance for the field. It is primarily intended for the pharmacy student, to teach the requirements in "analytical chemistry" for the 5 years pharmacy curriculum, but the textbook is also intended for analytical chemists moving into the field of pharmaceutical analysis.
- Addresses the basic concepts, then establishes the foundations for the common analytical methods that are currently used in the quantitative and qualitative chemical analysis of pharmaceutical drugs
- Provides an understanding of common analytical techniques used in all areas of pharmaceutical development
- Suitable for a foundation course in chemical and pharmaceutical sciences
- Aimed at undergraduate students of degrees in Pharmaceutical Science/Chemistry Analytical Science/Chemistry, Forensic analysis
- Includes many illustrative examples
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Introduction to Pharmaceutical Chemical Analysis by Steen Honoré Hansen,Stig Pedersen-Bjergaard,Knut Rasmussen in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction to Pharmaceutical Analysis
This chapter briefly reviews the life of medical products and the manufacture of medical products according to international regulations and guidelines. Based on this review the major areas and usage of pharmaceutical analysis are identified.
1.1 Applications and Definitions
The European Pharmacopeia defines a medical product as:
(a) Any substance or combination of substances presented as having properties for treating or preventing disease in human beings and/or animals; or (b) any substance or combination of substances that may be used in or administered to human beings and/or animals with a view either to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis.
A medical product contains a substance that is pharmacologically active and that substance is called the active ingredient (AI) or active pharmaceutical ingredient (API) defined as follows:
Any substance intended to be used in the manufacture of a medicinal product and that, when so used, becomes an active ingredient of the medicinal product. Such substances are intended to furnish a pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment or prevention of disease, or to affect the structure and function of the body.
An herbal medical product is:
A medicinal product, exclusively containing as active ingredients one or more herbal drugs or one or more herbal drug preparations, or one or more such herbal drugs in combination with one or more such herbal drug preparation.
Drug substances are administered very rare as the pure active substance. Typically the active substance and excipients (auxiliary substances) are combined into dosage forms to produce the final medical product. An excipient is:
Any constituent of a medicinal product that is not an active substance.
Adjuvants, stabilizers, antimicrobial preservatives, diluents, antioxidants, for example, are excipients.
The dosage form can be, for example, a tablet or a capsule or syrup to be administered orally, injections that are for parenteral administration into the body, or ointments for topical administration. Figure 1.1 shows typical dosage forms.
Figure 1.1 Different dosage forms
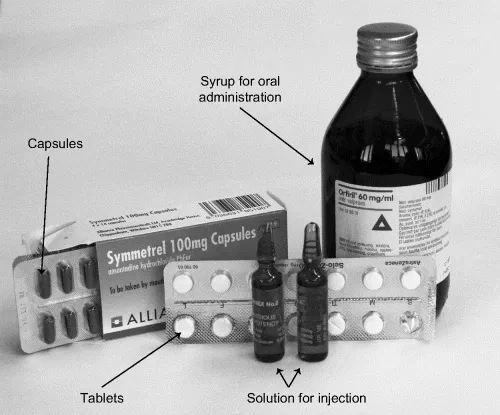
Formulation is the process in which different chemical substances, including the active ingredient and excipients are combined to produce a final medical product. It involves developing a preparation of the drug that is both stable and acceptable to the patient. For orally taken drugs this usually involves incorporating the drug and excipients in a solid dosage form such as a tablet or a capsule or a liquid dosage forms such as a syrup. The main function of excipients is summarized as follows:
- Ensure that the preparation has a shape and size that is easy to use for the patient;
- Ensure that the active substance is optimally adsorbed in the patient;
- Ensure that the preparation has an acceptable shelf life;
- Ensure that the preparation does not have an unpleasant taste or odor;
- Ensure easy production.
There is a wide spectrum of different excipients, which varies widely from preparation to preparation. To illustrate this, Table 1.1 shows the excipients of a tablet and syrup which both contain paracetamol as the active ingredient. Paracetamol is both an analgesic (an = no, algesis = pain) and a antipyretic (anti = against, pyretos = fever), which means that it is used against pain and fever.
Table 1.1 Excipients of a paracetamol tablet and a paracetamol syrup.
| Content | Amount (mg) | Function |
| Tablet (weight 285 mg) | ||
| Paracetamol | 250 | Active ingredient |
| Hydroxypropyl cellulose | Binder | |
| Maize starch | Disintegrant | |
| Talcum | Glidant | |
| Magnesium stearate | Lubricant | |
| Syrup (volume 1 ml) | ||
| Paracetamol | 24 | Active ingredient |
| Sorbitol | Sweetener | |
| Glycerol | Sweetener | |
| Polyvinylpyrrolidone | Thickening agent | |
| Saccharine sodium salt | Sweetener | |
| Methylparabene | Preservative | |
| Ethylparabene | Preservative | |
| Propylparabene | Preservative | |
| Sodium metabisulfite | Antioxidant | |
| Citric acid | pH regulator | |
| Sodium citrate | pH regulator | |
| Strawberry aroma | Flavoring agent | |
| Water | Solvent | |
The tablets, which in this example, have a total weight of 285 mg contains 250 mg of paracetamol (active ingredient), while the remaining 35 mg is made up of excipients. The excipients are a disintegrating agent, a lubricant, a glidant and a binder. Binders, lubricating and gliding agents are added to facilitate manufacture. A disintegrating agent ensures rapid disintegration of the tablet in the stomach.
Paracetamol syrup, which contains 24 mg/ml of paracetamol, is composed mainly of water. In addition, it is added sweetening and flavoring agents for better taste. Antimicrobial preservatives and antioxidants are added to prevent bacterial growth and chemical degradation. In addition agents that increase the viscosity and stabilizes the pH are added.
Medical products may be divided into over the counter drugs (OTC), which may be sold directly to the consumer in pharmacies and supermarkets without restrictions, and prescription only medicine (POM) that must be prescribed by a licensed practitioner. Medical products are predominantly produced by the pharmaceutical companies, only in rare occasions are pharmaceutical products produced in hospitals and in pharmacies. New products are often patented to give the developer exclusive right to produce them. Those that are not patented or with expired patents, are called generic drugs since they can be produced by other companies without restrictions or licenses from the patent holder. According to the European Federation of Pharmaceutical Industries and Associations (EFPIA), the pharmaceutical industry in Europe employed some 630 000 people, including 110 000 in research and development, in 2009. The trade surplus was Euro 55 200 million, and Euro 26 000 million was spent on pharmaceutical research and development. The retail value of the pharmaceutical market was Euro 215 000 million, which is just under 30% of the world market.
1.2 The Life of Medicines
Figure 1.2 outlines a typical industrial production of a pharmaceutical product.
Figure 1.2 Illustration of the manufacturing of a medical product
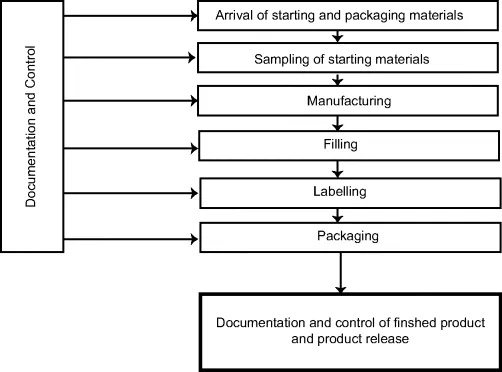
Production starts by ordering the current active ingredient and the necessary starting materials. In some cases, the company produces some of the ingredients, but most commonly they are produced elsewhere by various industrial raw material suppliers. The raw materials arrive in relatively large quantities (1–500 kg) and are typically packed in cardboard drums or in plastic containers. Figure 1.3 shows an example of a received batch of raw material in the photo gallery from a manufacturing facility.
Figure 1.3 Photo gallery of a manufacturing facility: arrival of raw materials, weighing, sampling, tablet pressing, filling, labelling, and packaging. Reproduced with permission from Fagbokforlaget






Figure 1.4 shows an outline of some areas found...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Chapter 1: Introduction to Pharmaceutical Analysis
- Chapter 2: International Pharmacopoeias, Regulations and Guidelines
- Chapter 3: Fundamental Chemical Properties, Buffers and pH
- Chapter 4: Fundamentals of Pharmaceutical Analysis
- Chapter 5: Titrimetric Methods
- Chapter 6: Introduction to Spectroscopic Methods
- Chapter 7: UV Spectrophotometry
- Chapter 8: IR Spectrophotometry
- Chapter 9: Atomic Spectrometry
- Chapter 10: Chromatography
- Chapter 11: Chromatographic Separation Principles
- Chapter 12: Thin-Layer Chromatography
- Chapter 13: High Performance Liquid Chromatography
- Chapter 14: Gas Chromatography
- Chapter 15: Capillary Electrophoresis
- Chapter 16: Mass Spectrometry
- Chapter 17: Miscellaneous Chemical Techniques
- Chapter 18: Sample Preparation
- Chapter 19: Analytical Chemical Characteristics of Selected Drug Substances
- Chapter 20: Quantification and Quality of Analytical Data
- Chapter 21: Chemical Analysis of Drug Substances
- Chapter 22: Chemical Analysis of Final Pharmaceutical Products
- Chapter 23: Analysis of Drugs in Biological Fluids
- Index