![]()
PART I
INTRODUCTION
![]()
CHAPTER 1
INTRODUCTION TO PROTEIN PURIFICATION
BO ERSSON
Medicago AB, Danmark-Berga 13, SE-755 98 Uppsala, Sweden
LARS RYDÉN
Centre for Sustainable Development (CSD) Uppsala, Uppsala University, Villavägen 16, SE-752 36 Uppsala, Sweden
JAN-CHRISTER JANSON
Department of Physical and Analytical Chemistry, Uppsala University, Box 579, S-751 23 Uppsala, Sweden
1.1 Introduction
1.2 The Protein Extract
1.2.1 Choice of Raw Material
1.2.2 Extraction Methods
1.2.3 Extraction Medium
1.2.3.1 pH
1.2.3.2 Buffer Salts
1.2.3.3 Detergents and Chaotropic Agents
1.2.3.4 Reducing Agents
1.2.3.5 Chelators or Metal Ions
1.2.3.6 Proteolytic Inhibitors
1.2.3.7 Bacteriostatics
1.3 An Overview of Fractionation Techniques
1.3.1 Precipitation
1.3.2 Electrophoresis
1.3.3 Chromatography
1.3.4 Expanded Bed Adsorption
1.3.5 Membrane Adsorption
1.4 Fractionation Strategies
1.4.1 Introductory Comments
1.4.2 Initial Fractionation
1.4.2.1 Clarification by Centrifugation and/or Microfiltration
1.4.2.2 Ultrafiltration
1.4.2.3 Precipitation
1.4.2.4 Liquid-Liquid Phase Extraction
1.4.3 The Chromatographic Steps
1.4.3.1 Choice of Adsorbent
1.4.3.2 The Order of the Chromatographic Steps
1.4.4 The Final Step
1.5 Monitoring the Fractionation
1.5.1 Assay of Biological Activity
1.5.2 Determination of Protein Content
1.5.3 Analytical Gel Electrophoresis
1.6 The Final Product
1.6.1 Buffer Exchange
1.6.2 Concentration
1.6.3 Drying
1.7 Laboratory Equipment
1.7.1 General Equipment
1.7.2 Equipment for Homogenization
1.7.3 Equipment for Chromatography
1.7.3.1 Column Design
1.7.3.2 Pumps and Fraction Collectors
1.7.3.3 Monitoring Equipment
1.7.3.4 Chromatography Systems
1.7.4 Equipment for Chromatographic and Electrophoretic Analyses
1.8 References
1.1 INTRODUCTION
The development of techniques and methods for the separation and purification of biological macromolecules such as proteins has been an important prerequisite for many of the advancements made in bioscience and biotechnology over the past five decades. Improvements in materials, utilization of computerized instruments, and an increased use of in vivo tagging have made protein separations more predictable and controllable, although many still consider purification of non-tagged proteins more an art than a science. However, gone are the days when an investigator had to spend months in search of an efficient route to purify an enzyme or hormone from a cell extract. This is a consequence of the development of new generations of chromatographic media with increased efficiency and selectivity as well as of new automated chromatographic systems supplied with sophisticated interactive software packages and data bases. New electrophoresis techniques and systems for fast analysis of protein composition and purity have also contributed to increasing the efficiency of the evaluation phase of the purification process.
In the field of chromatography, the development of new porous resin supports, new crosslinked beaded agaroses, and new bonded porous silicas has enabled rapid growth in high resolution techniques (high performance liquid chromatography, HPLC; fast protein liquid chromatography, FPLC), both on an analytical and laboratory preparative scale as well as for industrial chromatography in columns with bed volumes of several hundred liters. Expanded bed adsorption enables rapid isolation of target proteins, directly from whole cell cultures or cell homogenates. Another field of increasing importance is micropreparative chromatography, a consequence of modern methods for amino acid and sequence analysis requiring submicrogram samples. The data obtained are efficiently exploited by recombinant DNA technology, and biological activities previously not amenable to proper biochemical study can now be ascribed to identifiable proteins and peptides.
A wide variety of chromatographic column packing materials such as gel-filtration media, ion exchangers, reversed phase packings, hydrophobic interaction adsorbents, and affinity chromatography adsorbents are today commercially available. These are identified as large diameter media (90–100 μm), medium diameter media (30–50 μm) and small diameter media (5–10 μm) in order to satisfy the different requirements of efficiency, capacity, and cost.
However, not all problems in protein purification are solved by the acquisition of sophisticated laboratory equipment and column packings that give high selectivity and efficiency. Difficulties still remain in finding optimum conditions for protein extraction and sample pretreatment, as well as in choosing suitable methods for monitoring protein concentration and biological activity. These problems will be discussed in this introductory chapter. There will also be an overview of different protein separation techniques and their principles of operation. In subsequent chapters, each individual technique will be discussed in more detail. Finally, some basic equipment necessary for efficient protein purification work will be described in this chapter.
Several useful books covering protein separation and purification from different points of view are available on the market or in libraries (1–3). In “Methods of Enzymology,” for example, in older volumes 22, 34, 104, and 182 (4–7), but particularly in the most recent volume, 463 (8), a number of very useful reviews and detailed application reports will be found. The booklets available from manufacturers regarding their separation equipment and media can also be helpful by providing detailed information regarding their products.
1.2 THE PROTEIN EXTRACT
1.2.1 Choice of Raw Material
In most cases, interest is focused on one particular biological activity, such as that of an enzyme, and the origin of this activity is often of little importance. Great care should therefore be taken in the selection of a suitable source. Among different sources there might be considerable variation with respect to the concentration of the enzyme, the availability and cost of the raw material, the stability of the enzyme, the presence of interfering activities and proteins, and difficulties in handling a particular raw material. Very often it is compelling to choose a particular source because it has been described previously in the literature. However, sometimes it is advantageous to consider an alternative choice.
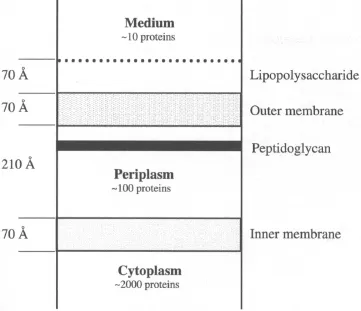
Traditional animal or microbial sources have today, to a large degree, been replaced by genetically engineered microorganisms or cultured eukaryotic cells. Protein products of eukaryotic orgin, cloned and expressed in bacteria such as Escherichia coli, may either be located in the cytoplasm or secreted through the cell membrane. In the latter case they are either collected inside the periplasmic space or they are truly extracellular, secreted to the culture medium. Proteins that accumulate inside the periplasmic space may be selectively released either into the growth medium by changing the growth conditions (9), or following cell harvesting and washing of the resuspended cell paste. At this stage, a considerable degree of purification has already been achieved by choosing a secreting strain as illustrated in Figure 1.1. In connection with the cloning, the recombinant protein may be equipped with an “affinity handle” such as a His-tag or a fusion protein such as Protein A, glutathione-S-transferase, or maltose binding protein in order to facilitate purification. The handle is often designed such that it can be cleaved off using highly specific proteolytic enzymes. Proteins of eukaryotic origin, and some virus surface proteins are often glycosylated why eukaryotic host cells have to be chosen for their production.
1.2.2 Extraction Methods
Some biological materials themselves constitute a clear or nearly clear protein solution suitable for direct application to chromatography columns after centrifugation or filtration. Examples include blood serum, urine, milk, snake venoms, and—perhaps most importantly—the extracellular medium after cultivation of microorganisms and mammalian cells, as mentioned above. It is normally an advantage to choose such a starting material because of the limited number of components and also because extracellular proteins are comparatively stable. Some samples, such as urine or cell culture supernatants, are normally concentrated before purification begins.
In most cases, however, it is necessary to extract the activity from a tissue or a cell paste. This means that a considerable number of contaminating molecular species are set free, and proteolytic activity will make the preparation work more difficult. The extraction of a particular protein from a solid source often involves a compromise between recovery and purity. Optimization of extraction conditions should favor the release of the desired protein and leave difficult-to-remove contaminants behind. Of particular concern is to find conditions under which the already extracted protein is not degraded or denatured while more is being released.
Various methods are available for the homogenization of cells or tissues. For further details and discussions the reader is referred to the paper by Kula and Schütte (10). The extraction conditions are optimized by systematic variation of parameters such as the composition of the extraction medium (see below), time, temperature, and type of equipment used.
The proper design of an extraction method thus requires preliminary experiments in which aliquots are taken at various time intervals and analyzed for activity and protein content. The number of parameters can be very large, so this part of the work has to be kept within limits by applying proper judgment. However, it is not recommended to accept a single successful experiment. Further investigations of the required extraction time, in part...