
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The efficacy of isocyanide reactions in the synthesis of natural or naturallike products has resulted in a renaissance of isocyanide chemistry. Now isocyanides are widely used in different branches of organic, inorganic, coordination, combinatorial and medicinal chemistry.
This invaluable reference is the only book to cover the topic in such depth, presenting all aspects of synthetic isonitrile chemistry. The highly
experienced and internationally renowned editor has brought together an equally distinguished team of authors who cover multicomponent
reactions, isonitriles in total synthesis, isonitriles in polymer chemistry and much more.
This invaluable reference is the only book to cover the topic in such depth, presenting all aspects of synthetic isonitrile chemistry. The highly
experienced and internationally renowned editor has brought together an equally distinguished team of authors who cover multicomponent
reactions, isonitriles in total synthesis, isonitriles in polymer chemistry and much more.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Isocyanide Chemistry by V. Nenajdenko in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Chiral Nonracemic Isocyanides
1.1 Introduction
Although isocyanides have proven to be very useful synthetic intermediates – especially in the field of multicomponent reactions – most research investigations performed to date on isocyanides have involved commercially available, unfunctionalized and achiral (or chiral racemic) compounds. Two reasons can be envisioned for the infrequent use of enantiomerically pure isocyanides: (i) the general lack of asymmetric induction produced by them; and (ii) the high tendency to lose stereochemical integrity in some particular classes of isonitriles. However, it is believed that when these drawbacks are overcome, the use of chiral non-racemic isocyanides in multicomponent reactions can be very precious, allowing a more thorough exploration of diversity (in particular stereochemical diversity) in the final products. Recently, several reports have been made describing the preparation and use of new classes of functionalized chiral isocyanides. In fact, several chiral isocyanides may be found in nature, and these will be briefly described in Section 1.5, with attention focused on their total syntheses. Another growing application of chiral isocyanides is in the synthesis of chiral helical polyisocyanides.
It is hoped that this review will encourage chemists first to synthesize a larger number of chiral isocyanides, and subsequently to exploit them in multicomponent reactions, in total synthesis, and in the material sciences.
1.2 Simple Unfunctionalized Isocyanides
The standard method used to prepare chiral isocyanides (whether functionalized, or not) begins from the corresponding amines, and employs a two-step sequence of formylation and dehydration (Scheme 1.1). Many enantiomerically pure amines are easily available from natural sources, classical resolution [1], or asymmetric synthesis. Formylation is commonly achieved via four general methods: (i) refluxing the amine in ethyl formate [2]; (ii) reacting the amine with the mixed formic–acetic anhydride [2]; (iii) reacting the amine with formic acid and DCC (dicyclohexylcarbodiimide) [3] or other carbodiimides [4]; and (iv) reacting the amine with an activated formic ester, such as cyanomethyl formate [5], p-nitrophenyl formate [6], or 2,4,5-trichlorophenyl formate [7]. For the dehydration step, several reagents are available, with the commonest and mildest methods involving POCl3, diphosgene, or triphosgene at low temperatures in the presence of a tertiary amine [2]. Although less commonly used, Burgess reagent (methyl N-(triethylammoniumsulfonyl)carbamate) [8] and the CCl4/PPh3/Et3N system [7] have also been employed.
Scheme 1.1
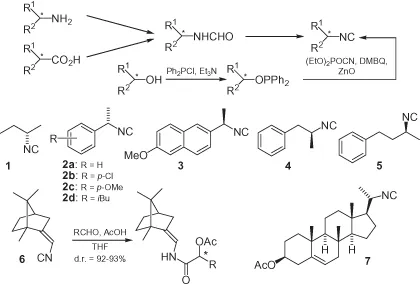
Alternatively, formamides can be obtained from chiral carboxylic acids, through a stereospecific Curtius rearrangement followed by reduction of the resulting isocyanate [9, 10].
Isocyanides may also be prepared from alcohols, by conversion of the alcohol into a sulfonate or halide, followed by SN2 substitution with AgCN [11]; however, this method works well only with primary alcohols. In contrast, a series of chiral isocyanides have been synthesized from chiral secondary alcohols via a two-step protocol that involves conversion first into diphenylphosphinite, followed by a stereospecific substitution that proceeds with a complete inversion of configuration [12]. The substitution step is indeed an oxidation–substitution, that employs dimethyl-1,4-benzoquinone (DMBQ) as a stoichiometric oxidant and ZnO as an additive. Alternatively, primary or secondary alcohols can be converted into formamides through the corresponding alkyl azides and amines.
Some examples of simple chiral isocyanides are shown in Scheme 1.1. These materials have all been prepared in a traditional manner, starting from chiral amines; the exception here is 5, which was synthesized from the secondary alcohol. The compounds comprise fully aliphatic examples such as 1 [13], α-substituted benzyl isocyanides such as 2 [1, 2, 13, 14] and 3 [14, 15], and α-substituted phenethyl or phenylpropyl isocyanides such as 4 [2] and 5 [12].
Because of the great synthetic importance of isocyanide-based multicomponent reactions, these chiral isocyanides have been often used as inputs in these reactions. The use of enantiomerically pure isocyanides can, in principle, bring about two advantages: (i) the possibility to obtain a stereochemically diverse adduct, controlling the absolute configuration of the starting isonitrile; and (ii) the possibility to induce diastereoselection in the multicomponent reaction. With regards to the second of these benefits, the results have been often disappointing, most likely because of the relative unbulkiness of this functional group. For example, Seebach has screened a series of chiral isocyanides, including 2a and 4 in the TiCl4-mediated addition to aldehydes, but with no diastereoselection at all [2]. This behavior seems quite general also for the functionalized isocyanides described later, the only exception known to date being represented by the camphor-derived isocyanide 6 [16], which afforded good levels of diastereoselection in Passerini reactions. The same isonitrile gave no asymmetric induction in the corresponding Ugi reaction, however. Steroidal isocyanides have also been reported (i.e., 7) [17, 18].
Apart from multicomponent reactions, and the synthesis of polyisocyanides (see Section 1.6), chiral unfunctionalized isocyanides have been used as intermediates in the synthesis of chiral nitriles, exploiting the stereospecific (retention) rearrangement of isocyanides into nitriles under flash vacuum pyrolysis conditions (FVP) [14, 19]. This methodology was used for the enantioselective synthesis of the anti-inflammatory drugs ibuprofen and naproxen, from 2d and 3, respectively. As isocyanides are usually prepared from amines, the overall sequence represents the homologation of an amine to a carboxylic derivative, and is therefore opposite to the Curtius rearrangement.
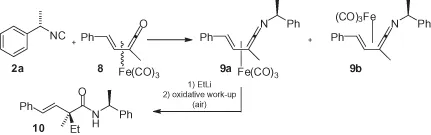
Another interesting application of 2a, as a chiral auxiliary, was reported by Alcock et al. (Scheme 1.2). Here, the chiral isocyanide reacts with racemic vinylketene tricarbonyliron(0) complex 8 to produce two diastereomeric (vinylketeneimine)tricarbonyliron complexes 9 that can be separated. Subsequent reaction with an organolithium reagent, followed by an oxidative work-up, was found to be highly diastereoselective, forming only adduct 10. This represents a useful method for accessing quaternary stereogenic centers, with the induction being clearly due to the tricarbonyliron group, while the isocyanide chirality serves only as a means of separating the two axial stereoisomers 9a and 9b [15].
Scheme 1.2
1.3 Isocyanides Containing Carboxylic, Sulfonyl, or Phosphonyl Groups
As the reactivity of α-isocyano esters and amides is reviewed in Chapters 3 and 4 of this book, attention at this point will be focused only on stereochemical issues; reactions exploiting reactivity at the α position will not be described.
1.3.1 α-Isocyano Esters
Enantiomerically pure α-isocyano esters 12 can be prepared by the dehydration of formamides 11, which in turn are synthesized in two steps from the corresponding α-amino acids [20, 21] (Scheme 1.3). The most critical step is dehydration, which has been demonstrated in some instances to be partly racemizing. The combination of diphosgene with N-methylmorpholine (NMM) at a low (<−25 °C) temperature has been reported in various studies to be able to avoid racemization and to be superior to the use of POCl3 with more basic amines [2, 22–25]. In a recent extensive study, the use of triphosgene/NMM at −30 °C was suggested as the method of choice [26], although a direct comparison of triphosgene with diphosgene was not carried out.
Scheme 1.3

These isocyanides would be very useful in multicomponent reactions, such as the Passerini and Ugi condensations, for the straightforward preparation of depsipetides or peptides, although racemization may be a relevant issue. Under Passerini conditions, these compounds appear to be configurationally stable during reaction with various aldehydes [22, 27–29], and this approach has been used, for example, in the total synthesis of eurystatin A [22] (Scheme 1.3). The quite complex tripeptide 16 has been assembled in just two steps by using a PADAM (Passerini–Amine Deprotection–Acyl Migration) strategy [3...
Table of contents
- Cover
- Related Titles
- Title page
- Copyright page
- Preface
- List of Contributors
- 1 Chiral Nonracemic Isocyanides
- 2 General Aspects of Isocyanide Reactivity
- 3 α-Acidic Isocyanides in Multicomponent Chemistry
- 4 Synthetic Application of Isocyanoacetic Acid Derivatives
- 5 Ugi and Passerini Reactions with Carboxylic Acid Surrogates
- 6 Amine (Imine) Component Surrogates in the Ugi Reaction and Related Isocyanide-Based Multicomponent Reactions
- 7 Multiple Multicomponent Reactions with Isocyanides
- 8 Zwitterions and Zwitterion-Trapping Agents in Isocyanide Chemistry
- 9 Recent Progress in Nonclassical Isocyanide-Based MCRs
- 10 Applications of Isocyanides in IMCRs for the Rapid Generation of Molecular Diversity
- 11 Synthesis of Pyrroles and Their Derivatives from Isocyanides
- 12 Isocyanide-Based Multicomponent Reactions towards Benzodiazepines
- 13 Applications of Isocyanides in the Synthesis of Heterocycles
- 14 Renaissance of Isocyanoarenes as Ligands in Low-Valent Organometallics
- 15 Carbene Complexes Derived from Metal-Bound Isocyanides: Recent Advances
- 16 Polyisocyanides
- Index