
eBook - ePub
Supported Ionic Liquids
Fundamentals and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Supported Ionic Liquids
Fundamentals and Applications
About this book
This unique book gives a timely overview about the fundamentals and applications of supported ionic liquids in modern organic synthesis. It introduces the concept and synthesis of SILP materials and presents important applications in the field of catalysis (e.g. hydroformylation, hydrogenation, coupling reactions, fine chemical synthesis) as well as energy technology and gas separation. Written by pioneers in the field, this book is an invaluable reference book for organic chemists in academia or industry.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Supported Ionic Liquids by Rasmus Fehrmann, Anders Riisager, Marco Haumann, Rasmus Fehrmann,Anders Riisager,Marco Haumann in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
1.1 A Century of Supported Liquids
Natural and synthesized solid materials are generally characterized by a nonuniform and undefined surface. The surface contains face atoms, corner atoms, edge atoms, ad-atoms, and defect sites, which together determine the surface properties of the material [1]. In many applications, these different sites display different properties, for example, with respect to their chemical activity. Often, only certain sites are advantageous with regard to the specific application of the material as in the case of, heterogeneous catalysts and adsorbents. Future development of more efficient catalysts and adsorbents in industrial processes will depend on the design of solid surfaces that allow all surface atoms to be most effective. At the same time, new technologies are required, which will lead to the design of completely new surface properties within solids [2].
One possible way to achieve a uniform surface is by coating the solid support material with a thin liquid film, thereby defining the material properties by the liquid's properties. Such supported liquid phase (SLP) materials date back a 100 years ago till 1914, when BASF introduced a silica-supported V2O5-alkali/pyrosulfate SO2 oxidation catalyst for sulfuric acid production (see Figure 1.1) [3]. This catalyst, which is still the standard system for sulfuric acid production today, can be described as a supported molten salt, as it consists of a mixture of vanadium alkali sulfate/hydrogensulfate/pyrosulfate complexes that are present under reaction conditions (400–600 °C) [4].
Figure 1.1 Historical development of supported liquids in catalysis.

The concept of supported liquid catalysis is not restricted to liquid salts. In order to apply the concept of uniform surface properties and efficient catalyst immobilization, several authors investigated the SLP concept during the 1970s and 1980s [5–11]. However, later studies revealed that the evaporation of the loaded liquid cannot be avoided completely during operation. This is especially a problem when using water as the liquid phase [12–17]. In these supported aqueous phase (SAP) systems, the thin film of water evaporated quickly under reaction conditions, making the concept applicable only for slurry-phase reactions with hydrophobic reaction mixtures.
1.2 Supported Ionic Liquids
The supported ionic liquid phase (SILP) technology is a fundamental, new approach to obtain liquid containing solid materials that do not evaporate, made through surface modification of a porous solid by dispersing a thin film of ionic liquid (IL) onto it, as depicted in Figure 1.2 [18, 19]. ILs are salts consisting completely of organic cations and inorganic or organic anions (for further details see Chapter 2) [20]. Their better charge distribution and larger ion size compared to classical inorganic salts result in melting points below 100 °C. Owing to the extremely low vapor pressure of ILs, the surface of SILP materials is coated permanently, even under elevated reaction conditions. By variation of anions and cations, solubility, reactivity, and coordination properties of the ILs can be changed according to the special requirements of the given application.
Figure 1.2 Schematic representation of an ionic liquid film supported on a porous material.
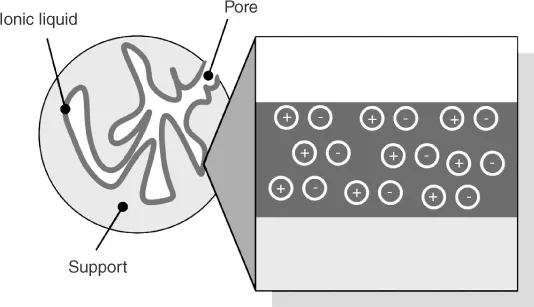
With respect to material and surface design, ILs are characterized by a highly pre-organized, homogeneous liquid structure with distinctive physicochemical characteristics and these – often unique – characteristics are exclusively governed by the combination of ions in the material [20]. Hence, by an appropriate choice of the ions (and eventually additives) contained in the IL material, it is possible to transfer specific properties of the fluid to the surface of a solid material by confining the fluid to the surface. Thus, the SILP concept allows custom-making of solid materials, resulting in uniform and well-defined surface topologies with definite properties and a controlled chemical reactivity. Importantly, the SILP concept thereby constitutes an attractive methodology to circumvent the lack of uniformity of solids in traditional material science. In addition, the approach provides a great potential to create materials with new surface properties, as the transfer of specific IL properties to solid surfaces may result in “designer surfaces” with properties that are impossible to realize with any present synthetic approach.
In principle, all ILs can be contacted with a solid surface and therefore, looking at the tremendous numbers of publications in the field of “ILs,” exceeding 6700 in the year 2012, it is anticipated that the concept of “supported ILs” will benefit from this scientific input.1
A common method to immobilize ILs on surfaces is the covalent anchoring of a monolayer of IL onto a support – usually pretreated – as shown in Figure 1.3a. Here, the IL becomes part of the support material, thereby losing certain bulk phase properties such as solvation strength, conductivity, and viscosity. The IL can contain a certain functionality (e.g., acidity, hydrophobicity) that will render the support surface.
Figure 1.3 Categorization of materials based on supported ionic liquid films according to the phase behavior of the supported ionic liquid: (a) covalently attached monolayer and (b) multilayers of ionic liquid.
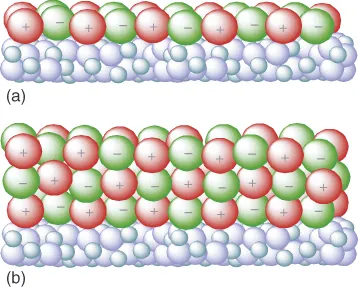
If multilayers of IL are immobilized onto a support, the bulk properties of the IL can be retained. In such SILP systems, depicted schematically in Figure 1.3b, functionalities can be incorporated by dissolving, for example, metal salts, acids, transition metal complexes, and nanoparticles.
Various efficient and recyclable systems based on the latter category have been developed, including supported ionic liquid catalysis (SILC), supported ionic liquid catalysts (SILCA), solid catalyst with ionic liquid (SCIL), solid catalysts with ionic liquid layer (SCILL), supported ionic liquid nanoparticles (SILnPs), supported ionic liquid phase (SILP), supported ionic liquid phase catalyst (SILPC), ionic liquid crystalline-SILP (ILC-SILP), structured SILP (SSILP), supported ionic liquid-like phase (SILLP), polymer-supported ionic liquid (PSIL), and supported ionic liquid membrane (SILM). All of these concepts try to use the intrinsic properties of IL bulk phases and can be regarded as derivatives of the general SILP concept, which itself is a branch of the “SLP-tree.”
The synthesis of SILP materials is usually straightforward and the thin film of IL is fixed on the surface mainly by physisorption, and in a few cases by chemisorption [21]. The IL is mixed with the support and the catalyst complex (if applied) in a low-boiling solvent. The solvent is then removed by evaporation or freeze-drying, yielding a dry, free-flowing powder as the SILP catalyst. Depending on the amount of IL and the pore structure of the support material, film thicknesses between 3 and 30 nm can be accomplished. Detailed descriptions of support materials and synthetic methodologies are given in Chapters 3 and 4 while the structure and stability of these materials are discussed in Chapters 5 and 6. Solid-state NMR studies of different amounts of IL on silica support indicated that below a critical value of 10 vol% IL loading, small islands of ILs exist on the support [22]. At values higher than 10 vol%, complete surface coverage with IL was observed, which resembled the characteristics of the bulk IL. This is an important prerequisite for the efficient immobilization of homogeneous catalyst complexes that would lose activity and, more importantly, selectivity upon interaction with the support surface or in a constrained environment. Spectroscopic studies of SILP materials are summarized in Chapters 7 and 8 while Chapter 9 introduces tools for a-priori selection of suitable ionic liquids.
Form an engineering point of view these SILP materials offer some advantages compared to classical gas–liquid or liquid–liquid systems, especially
- a high surface area supplied by the support structure
- a thin film of liquid that circumvents mass transport problems
- adjustable solvent properties, for example, solubility
- thermal stability of most ILs up to 200 °C
- application of fixed-bed or fluidized-bed reactor technology
- efficient catalyst immobilization in defined environment.
1.3 Applications in Catalysis
In SILP catalysis, work is focused mainly on the immobilization of homogeneous transition metal complexes within the thin IL film. Homogeneous catalysts, in contrast to their heterogeneous counterparts, have a uniform molecular structure and can easily be modified by the use of dedicated ligands in terms of reactivity, selectivity, and stability [23]. The main drawback of homogeneous catalysis is the elaborate recycling of the dissolved catalyst from the reaction mixture, usually accomplished by distillation or extraction. This issue, which currently limits more applications of homogeneous catalysts in continuous processes, can be circumvented by the SILP technology.
The use of SILP systems in catalysis has been reviewed recently, including both liquid and gas-phase applications [21, 24]. With respect to the application of these solid materials in liquid phase slurry reactions, the leaching of IL from the support is the most crucial issue. The smallest cross-solubility of the IL in the liquid substrate or product phase will cause rapid removal of the thin film accompanied by leaching of the catalyst complex, resulting in lower catalyst activity.
This problem can be circumvented in a very elegant manner if the reaction is performed in SILP gas-phase contact. Since the IL does not have any technically relevant vapor pressure, it is not removed via gas-phase leaching, and catalyst stabilities have been found to be very high [25]. Moreover, the gas-phase has no so...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Preface
- List of Contributors
- Chapter 1: Introduction
- Part I Concept and Building Blocks
- Part II Synthesis and Properties
- Part III Catalytic Applications
- Part IV Special Applications
- Part V Outlook
- Index