
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This is the first detailed description in English of radiation and polymeric material interaction and the influences of thermal and optical material properties. As such, it provides comprehensive information on material and process characteristics as well as applications regarding plastic laser welding.
The first part of this practical book introduces the structure and physical properties of plastics, before discussing the interaction of material and radiation in the NIR and IR spectral range. This is followed by an overview of the physical foundations of laser radiation and laser sources used for plastic welding. The third part describes the main processes of laser welding thermoplastics, as well as possibilities of process control, design of joint geometry, material compatibilities and adaptation of absorption of plastics to NIR radiation. Finally, the author explains applications of laser welding plastics using several industrial case studies from the automotive industry, household goods, and medical devices.
Tailored to the needs of everyone dealing with laser welding of plastics, especially engineers in packaging, component manufacturing, and the medical industry.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
- Polyaddition as chain reaction: Process by chemical combination of a large number of monomer molecules, in which the monomers will be combined to a chain either by orientation of the double bond or by ring splitting. No byproducts will be separated and no hydrogen atoms will be moved within the chain during the reaction. The process will be started by energy consumption (by light, heat or radiation) or by use of catalysts. Figure 1.1 Processes for generating plastics and examples [1].
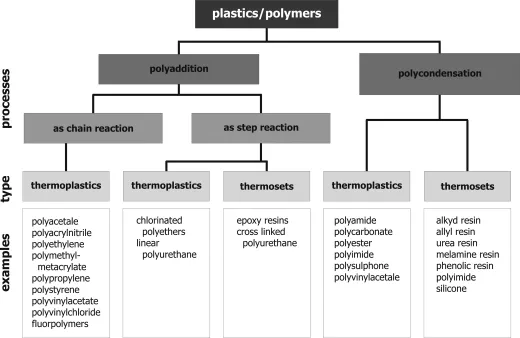
- Polyaddition as step reaction: Process by combination of monomer units without a reaction of double bonds or separation of low molecular compounds. Hydrogen atoms can change position during the process.
- Polycondensation: Generation of plastics by build up of polyfunctional compounds. Typical small molecules like water or ammonia can be set free during the reaction. The reaction can occur as a step reaction.
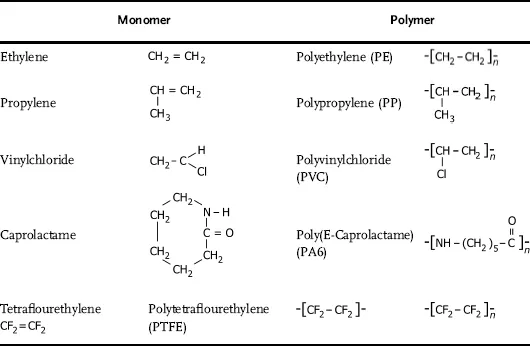
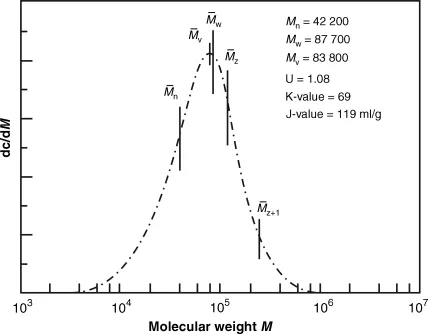
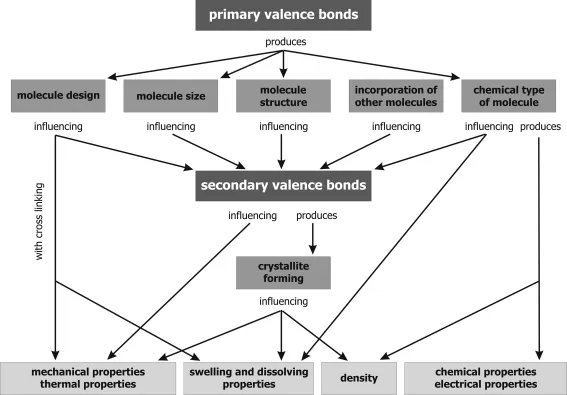
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Introduction
- Chapter 1: Material Properties of Plastics
- Chapter 2: Laser Sources for Plastic Welding
- Chapter 3: Basics of Laser Plastic Welding
- Chapter 4: Process of Laser Plastic Welding
- Chapter 5: Case Studies
- Index