![]()
1
Reaction Control by Molecular Recognition–A Survey from the Photochemical Perspective
Cheng Yang1, Chenfeng Ke2, Yu Liu2, and Yoshihisa Inoue1
1 PRESTO (JST) and Department of Applied Chemistry, Osaka University, Suita 565-0871, Japan2 Department of Chemistry, Nankai University, Tianjing, China
1.1 Introduction
Molecular recognition through non-covalent interactions between two or more molecules has attracted much attention from a broad spectrum of chemists for a long period of time and has already found many applications in various areas of science and technology. The concept of molecular recognition was first developed for biomolecular systems such as enzyme, antibody and DNA, which can selectively bind the specific target molecules through non-covalent weak interactions, including hydrogen bonding, van der Waals, dipole–dipole, charge–dipole and hydrophobic interactions.1–3 Recent studies on artificial host–guest systems have revealed that molecular recognition is the essential conceptual basis for supramolecular chemistry and nanotechnology.4,5
Reaction control through complexation of substrate by supramolecular host is a relatively new idea compared to the conventional approaches that involve simple collisional attack or coordination of substrate to metal. Multiple non-covalent interactions in supramolecular assembly bind and locate a site-specific substrate in the right position, orientation and conformation near the catalyst or active site, stabilize the high-energy transition state, and eventually make the reaction faster and more selective. Typical examples are found in enzymatic reactions, which proceed with high specificity and efficiency in aqueous solutions under mild conditions. These observations in natural systems have inspired researchers to develop novel research areas such as supramolecular chemistry, biomimetic chemistry and bio-inspired materials science and technology.6,7
Contrary to the thermal counterpart, photochemical reactions in supramolecular system have been less investigated and therefore of current interest. Photochemistry is a powerful tool in synthetic chemistry as a complementary method for achieving compounds that are difficult to obtain through thermal reactions due to high strain, low stability, and orbital symmetry reasons. Unlike thermal reactions, photoreactions deal with excited-state molecules that are usually short-lived but experience much lower energy barriers and exhibit high reactivities even at low temperatures. As a consequence of these features, the precise control of a photoreaction is more difficult to achieve than that of thermal one. This is one of the reasons why most asymmetric photoreactions result in only relatively low enantioselectivities. In this context, supramolecular approaches to the photochemical asymmetric synthesis enable the more precise control of the orientation and conformation of substrates and, as a result, the enantioselectivity of photoproducts, by utilizing the non-covalent interactions in both ground and excited states.
Supramolecular photochemistry is a relatively new interdisciplinary area of science and may be tracked back to the work in early 1980s, where the spectroscopic properties of ions were manipulated by crown ethers.8–10 The rapid development of supramolecular chemistry in the last two decades accelerated the application of supramolecular systems to organic photochemistry 11,12 and more recently to asymmetric synthesis, 13,14 leading to a great number of publications on reaction control by molecular recognition. Consequently, not all of these areas will be covered, but the concentration will be rather on the representative supramolecular photoreactions conducted primarily in solution. This will help idenitify the crucial concepts, strategies and conclusions as well as the major factors and mechanisms that govern the supramolecular photochemistry in different systems, and also provide the possible applications and future perspectives of this interesting area of supramolecular chemistry.
1.2 Photochemical Reactions Mediated by Macrocyclic Compounds
1.2.1 Supramolecular Photoreactions with Crown Ethers
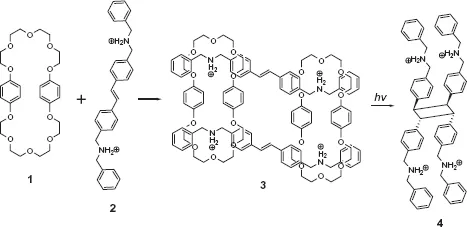
Crown ethers, a family of cyclic oligomers of ethylene oxide, are artificial macrocyclic hosts which have been synthesized and utilized since the early days of supramolecular chemistry.15,16 Besides various metal ions that are complexed by crown ethers mainly through ion–dipole interaction, primary and secondary organic ammonium ions also form stable complexes with larger sized crown ethers through ion–dipole and hydrogen-bonding interactions. Stoddart and co-workers used crown ethers that can simultaneously bind two organic ammonium guests to facilitate photodimerization. 2 forms a doubly encircled, doubly threaded 2: 2 complex with bis-p-phenylene-34-crown-10 1 to give a centrosymmetric [4]pseudorotaxane in the solid state. In addition to the hydrogen-bonding interactions between 1 and 2, the complex is also stabilized by π–π stacking interactions between the two trans-stilbene units with mean interplanar and centroid-centroid separations of 3.57 and 4.33 Å, respectively. The close arrangement of stilbenes accelerates the photodimerization upon irradiation to17 As illustrated in Scheme 1.1, trans-stilbene derivative exclusively give a single cyclobutane isomer 4 with a syn–anti–syn conformation, as confirmed by X-ray crystallographic analysis. A control experiment showed that no photodimerization but only trans-to-cis isomerization took place in the absence of crown ether 1.
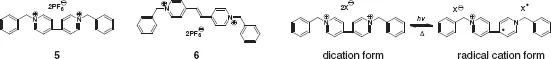
The photochromic behavior of viologens is greatly affected upon complexion with crown ether. Viologens show photoinduced colour change in the absence of any additional reagents when dispersed in isotropic thin polymer films.18 Irradiation of benzylviologen 5 (Scheme 1.2) incorporated in a polymer matrix caused a colour change from colourless to blue as a result of the reduction of 5 from dication to radical cation, which was reverted to dication within 2 h in the dark. Crown ether 1 forms a 1: 1 complex with 5 and also with 6 in acetone with association constants of ca. 200 M—1. The charge-transfer (CT) interaction between viologen 5 or 6 and 1 led to the formation of yellow-coloured CT complex with a CT absorption band at 453 nm and 421 nm for 5 and 6, respectively. The crown ether complexes of photoreduced viologens showed much accelerated bleaching rates than the corresponding free viologen radical cations. A similar phenomenon was observed also for 2 ⊂ 1 complex in a polymer matrix.
1.2.2 Supramolecular Photoreactions with Calixarenes
Calix[n]arenes are a class of macrocycles that are normally made up of phenol units linked with methylene bridges and possess cavities of various sizes that can accommodate small organic molecules primarily driven by hydrophobic interactions.Water soluble p-sulfonatocalix[6]arene (SCA[6]) and [8]arene (SCA[8]) (Scheme 1.3) have dimensions of 15.9 × 11.8 Å and 20.4 × 16.7 Å at the upper rim and 6.5 × 3.3 Å and 8.6 × 5.9 Å at the lower rim, respectively,19,20 and provide suitable cavities for accommodating quaternary ammonium ions, 21,22 native amino acids,23 and small neutral organic molecules.24 Using SCA[6] and SCA[8]25 as supramolecular hosts, Ramamurthy et al. investigated the photochemistry of benzoin alkyl ethers 7a–c.26 28– These SCAs formed the corresponding 1: 1 complexes with 7a–c in aqueous solution, and 7a showed modest association constants of 137 M—1 and 386 M—1 with SCA[6] and SCA[8], respectively. Upon photolysis, these benzoin alkyl ethers underwent both the Norrish type I (α -cleavage) and type II (γ-hydrogen abstraction) reactions to give photoproducts 8–12 (Scheme 1.3). In the absence of SCAs, pinacol ether 9 was obtained as in 92% relative yield in aqueous solution at pH 7, along with deoxybenzoin 11 (8% relative yield), for which the α-cleavage (kα ≈1010 s—1) faster than the γ-hydrogen abstraction (kγ ≈109 s—1) is responsible. However, when the photoreaction was performed in the presence of SCA, product 11 became the major product. The relative yield of the type II products 11 and 12 depends critically on the -cavity size of hosts and association constants. Photoirradiation of 7a in the presence of an 8-fold excess amount of SCA[8] o...