
eBook - ePub
Optically Stimulated Luminescence
Fundamentals and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Optically Stimulated Luminescence
Fundamentals and Applications
About this book
Optically stimulated luminescence has developed into one of the leading optical techniques for the measurement and detection of ionizing radiation. This text covers, in a readable manner, advanced modern applications of the technique, how it can play a useful role in different areas of dosimetry and how to approach the challenges presented when working with optically stimulated luminescence.
The six chapters are as follows:
- Introduction, including a short history of OSL and details of successful applications
- Theory and Practical Aspects
- Personal Dosimetry
- Space Dosimetry
- Medical Dosimetry
- Other Applications and Concepts, including retrospective and accident dosimetry, environmental monitoring and UV dosimetry
Throughout the book, the underlying theory is discussed on an as-needed basis for a complete understanding of the phenomena, but with an emphasis of the practical applications of the technique. The authors also give background information and relevant key references on each method, inviting the reader to explore deeper into the subject independently.
Postgraduates, researchers, and those involved with radiation dosimetry will find this book particularly useful. The material is both relevant and accessible for both specialists and those new to the field, therefore is fundamental to any academic interested in modern advances of the subject.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Optically Stimulated Luminescence by Eduardo G. Yukihara,Stephen W. S. McKeever in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Atomic & Molecular Physics. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction
1.1 A Short History of Optically Stimulated Luminescence
Twelfthly, To satisfie my self, whether the Motion introduc’d into the Stone did generate the Light upon the account of its producing heat there, I held it near the Flame of a Candle, till it was qualify’d to shine pretty well in the Dark….
—Boyle, 1664
The readers of this book may find it unusual to start a text with the word “Twelfthly.” The above quotation is from a lively description of an experiment performed by Sir Robert Boyle, and the quoted text describes one (the 12th) of several experiments conducted by this seventeenth-century luminary on a piece of diamond loaned to him for the purpose. This prose, presented by Boyle to the Royal Society of London in October of 1663, concerns the phenomenon of thermoluminescence and Boyle’s colorful account is now widely regarded as the first written description of its observation (Boyle, 1664). Thermoluminescence. (TL) – also known (perhaps more accurately) as thermally stimulated luminescence – is one of a set of properties collectively known as “thermally stimulated phenomena. ” (Chen and McKeever, 1997). Boyle (1680), as cited by Bender and Marriman (2005), used the beautifully descriptive term “self-shining” to describe the phenomenon of luminescence. , but the modern term (and, indeed, the first use of the word “thermoluminescence”) is attributed to Eilhardt Wiedemann in his comprehensive studies of a variety of luminescence phenomena: “I have ventured to employ the term luminescence for all those phenomena of light which are more intense than corresponds to the actual temperature” (Wiedemann, 1889; quote from the Oxford English Dictionary, 1997 edition).
Thermoluminescence. (TL) refers to the process of stimulating, using thermal energy, the emission of luminescence from a substance following the absorption of energy from an external source by that substance. The usual source of external energy is ionizing radiation. and, as such, TL is closely related to phosphorescence. , which is the afterglow emitted from a substance after the absorption of external energy. (See Harvey (1957) for a comprehensive review of the early literature on this topic. A more modern discussion of TL and its relationship to phosphorescence can be found in Chen and McKeever (1997). Early studies of these phenomena were closely connected with the discovery of radioactivity and the external energy source in these early studies was invariably some form of ionizing radiation, from X-rays, an electron beam or a radioactive substance.
Optically stimulated luminescence. (OSL) is a related phenomenon in which the luminescence is stimulated by the absorption of optical energy, rather than thermal energy. It is difficult to identify when studies of OSL (or, as it is also known, photostimulated luminescence. , PSL) were first described in the literature. However, certainly the phenomenon was hinted at when initially Edmond Becquerel (1843) and then Henri Becquerel (1883) observed that the phosphorescence from zinc and calcium sulfides was quenched if these materials were exposed to infrared illumination after exposure to an ionizing radiation source. These and other similar observations around this time (Harvey, 1957) noted that the infrared illumination could either increase or decrease the intensity of the phosphorescence. Harvey (1957) reports that Henri Becquerel clearly observed an initial increase in luminescence output on application of the infrared light. The term “photophosphorescence” first appeared to describe these effects some years later (viz. 1889, as cited in The Century Dictionary, 1889 edition). Nichols and Merritt (1912) also noted that infrared stimulation can increase the luminescence output before rapidly quenching the phosphorescence and discussed the phenomenon in terms of Wiedemann and Schmidt’s “electric dissociation” theory, involving the separation of positive and negative charges induced by the absorption of radiation energy (Wiedemann and Schmidt, 1895). Already one can discern the glimmerings of the modern interpretation, which involves ionization of electrons from their parent atoms, despite the fact that these early ideas were formulated before the advent of quantum mechanics. and band structure theory..
At this point it is perhaps important to distinguish these effects from the property of “photoluminescence. .” The latter phenomenon describes prompt luminescence emission (or fluorescence) emitted during absorption of the stimulation light. No prior absorption of energy from an external ionizing source is necessary. A notable property of photoluminescence is that the emitted light is of a longer wavelength than that of the stimulation light. Furthermore, the lifetime of photoluminescence emission is such that it decays promptly upon cessation of the stimulation. Emission wavelengths in photophosphorescence, however, can be either longer or shorter than the stimulation wavelength, and the emission generally persists for seconds or minutes after the end of the stimulation period. Many examples of photophosphorescence are referred to in the early literature, but part of the difficulty in determining when reports of the phenomenon first appeared relates to the lack of understanding at that time of the physics of luminescence in general. As pointed out by Marfunin (1979), unlike other physical phenomena being studied in the early centuries, a complete understanding of luminescence requires an understanding of quantum mechanics, a field that was not born until the early decades of the twentieth century. Knowledge of quantized energy levels, band structure, and radiative and non-radiative electronic transitions was yet in the future. As a result critical experiments were perhaps not performed or the descriptions of them were vague such that easy identification of the phenomenon being studied is not always clear from the early literature.
Nevertheless, by the mid-twentieth century the understanding that free electrons in delocalized bands were involved in the phosphorescence process was beginning to emerge. As discussed by Leverenz (1950), a debate at that time concerned the connection between photoconductivity. and photophosphorescence. Work on sulfide materials (CdS, ZnS) demonstrated that the growth during stimulation and the decay after stimulation of both photoconductivity and photophophorescence were similar in many cases, although other materials seemed to show that a one-to-one connection was not always the case (e.g. Bube, 1951). Nevertheless, a picture emerged that photophosphorescence from those materials for which the luminescence decay was characterized by a t−n law (where t is time and n is usually between ∼0.5 and ∼2.0) required photostimulated conduction involving free charge carriers in conduction states.
Leverenz (1949) also discusses how infrared light can both quench the phosphorescence or stimulate it. Figure 1.1 illustrates a sequence of possible luminescence events in a complex phosphor made from Sr(S,Se):SrSO4:CaF2:Sm:Eu, as described by Leverenz (1949). Yellow luminescence is emitted during initial excitation with blue light, followed by a rapid decay (fluorescence, or photoluminescence) along with a component with a longer, slower decay (phosphorescence). However, if the material is then subsequently stimulated with infrared light, there is enhanced luminescence (growth and decay), the decay time for which can be rapidly reduced and the luminescence quenched by changing to shorter wavelength illumination (orange).
Figure 1.1 The sequence of luminescence emission from Sr(S,Se):SrSO4:CaF2:Sm:Eu. The luminescence during periods (c) and (d) are what we now term optically stimulated luminescence (Schematic redrawing of Figure 3 from Leverenz (1949).)
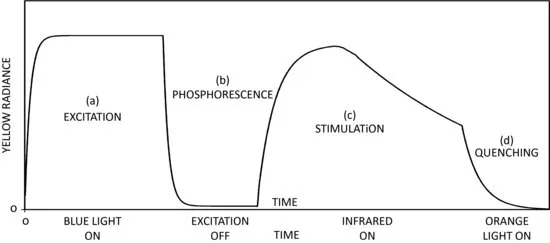
Even though the same emitting center is being activated in the sequence of luminescence emissions illustrated in Figure 1.1, the observed decay times can vary considerably, depending upon whether or not the sample is being optically stimulated and, if so, the intensity and wavelength(s) chosen. Leverenz (1949) notes: “The emitting center loses control over τ” (the luminescence decay time) “when the energy storage of phosphors consists of trapped excited electrons or metastable states, for then additional activation energy must be supplied to release the trapped electrons.” He goes on to state: “This activation energy may be supplied by heat … or it may be supplied by additional photons …” In these early descriptions of photophosphorescence can be found the essential elements of the phenomenon that we now term optically stimulated luminescence. Namely, after irradiation with the primary ionizing source, energy may be stored in the material in the form of trapped charge carriers (electrons and holes). Release of the trapped charge can then be stimulated by the absorption of optical photons of appropriate wavelength, resulting in luminescence emission. The emission decays with a time constant dictated by the wavelength and intensity of the stimulation light, and the characteristics of the trapping states in the material. This understanding of the processes involved was used by Schulman et al. (1951) and Mandeville and Albrecht (1953) to describe the luminescence emitted from alkali halides during optical stimulation following initial gamma irradiation, although OSL was not the term used by these authors. Indeed, nomenclature was still being developed, with Mandeville and Albrecht calling the effect “photostimulation phosphorescence. ,” or alternately “co-stimulation phosphorescence. ,” while Schulman et al. preferred the more descriptive (and more accurate) term “radiophotostimulation. .” Albrecht and Mandeville (1956) used the term “photostimulated emission” when describing what we now know as OSL from X-irradiated BeO. Harvey (1957) discusses the original 1843 observation of E. Becquerel and notes how this effect “could be called photo-stimulation, analogous to thermostimulation, that is thermoluminescence.” (See Table 1.1.)
Table 1.1 Some early nomenclature, along with the date of first introduction and author(s), for what is now known as optically stimulated luminescence.
| Description of the Phenomenon | Name of the Phenomenon | Author, and Date of First Introduction |
| Light stimulated transfer of electrons from deep traps to shallow traps followed by phosphorescence | Photophosphorescence Delayed optically stimulated luminescence | Unidentifieda (1889) Yoder and Salasky (1997) |
| Light stimulated release of electrons from deep traps followed by radiative recombination and subsequent luminescence | Radiophotostimulation Photostimulation phosphorescence Co-stimulation phosphorescence Photostimulated emission Optically stimulated luminescence | Schulman et al. (1951) Mandeville and Albrecht (1953) Mandeville and Albrecht (1953) Albrecht and Mandeville (1956) Fowler (1963) |
| a The term is first cited in The Century Dictionary, 1889 edition. | ||
Considering these similar terms used to describe the effect it is perhaps not surprising to discover that the first use of the modern term, optically stimulated luminescence OSL, appeared in the published literature a few years later. Fowler (1963) uses the term when describing a paper that is generally taken to be the first reported use of (what Fowler refers to as) OSL in radiation dosimetry. Antonov-Romanovsky et al. (1955) monitored the intensity of infrared-stimulated luminescence from various sulfides, after irradiation, and the intensity of the emitted light was used as a monitor of the dose of initial radiation. Although this is certainly one of the earliest examples of the use of OSL in radiation dosimetry, this now-famous 1956 paper refers to work by the same authors (published in Russian) from a few years earlier, in the 1949–1951 era. Nevertheless, despite these early applications, there was a hiatus of more than a decade before this pioneering work was followed by similar studies, notably by Braünlich, Schaffer and Scharmann (1967) and Sanborn and Beard (1967), working with irradiated sulfides. The work of this period even led to a US patent by Wallack (1959) in which was claimed the invention of an OSL gamma radiation dosimeter and system for reading the OSL signal using sulfide materials. Even then, however, OSL still did not catch on as a dosimetry tool; the cause lay in the materials being studied.
The fact that the sulfide materials used by these early pioneers could be stimulated with infrared (IR) light pointed to the fact that the trapped charge was localized in energy levels that were relatively shallow with respect to the delocalized bands, requiring a small de-trapping (activation) energy. This in turn meant that the trapped electrons were unstable at room temperature and decayed through the process of thermal stimulation (and subsequent phosphorescence emission). Thus, the dosimetric signal (i.e. the infrared stimulated luminescence signal) was found to decay with time between the initial absorption of radiation and the time of IR stimulation – a process now commonly referred to as “fading. .” As a result, OSL dosimetry was slow to be adopted, primarily for lack of suitable materials.
Slowly, however, the published literature began to accumulate descriptions of studies on optically stimulated luminescence effects in a variety of other material types. Most of the studies in this period (the 1970s, 1980s and even into the 1990s) reverted back to the use of photophosphorescence, as practitioners experimented with the optical stimulated transfer of electrons from deep, stable traps, into shallow, unstable traps. The goal was to monitor the subsequent phosphorescence as the charge leaked away from the shallow traps before recombining at the emission sites and to use this as a measure of absorbed dose. The materials used in this period, however, were wide-band-gap insulator. s, such as BeO (Rhyner and Miller, 1970; Tochilin, Goldstein and Miller, 1969), CaF2 (Bernhardt and Herforth, 1974), CaSO4 (Pradhan and Ayyanger, 1977; Pradhan and Bhatt, 1981) and Al2O3 (Yoder and Salasky, 1997). (The last work led to coining a new term for photophosphorescence, namely “delayed OSL. .”) Although photoconducting, narrow-band-gap material. s were still studied intensely, focus for dosimetry began to shift away from these materials to wide-band-gap insulating materials with deep, stable traps.
The major breakthrough for use of OSL in dosimetry emerged in a related but quite different area of science – in the wo...
Table of contents
- Cover
- Title Page
- Copyright
- Dedication
- Preface
- Acknowledgments
- Disclaimer
- List of Acronyms
- 1: Introduction
- 2: Theory and Practical Aspects
- 3: Personal Dosimetry
- 4: Space Dosimetry
- 5: Medical Dosimetry
- 6: Other Applications and Concepts
- References
- Index
- Color Plate