![]()
Section 1
Strength and Conditioning Biology
![]()
1.1
Skeletal Muscle Physiology
Valmor Tricoli
University of São Paulo, Department of Sport, School of Physical Education and Sport, São Paulo, Brazil
1.1.1 INTRODUCTION
The skeletal muscle is the human body’s most abundant tissue. There are over 660 muscles in the body corresponding to approximately 40–45% of its total mass (Brooks, Fahey and Baldwin, 2005; McArdle, Katch and Katch, 2007). It is estimated that 75% of skeletal muscle mass is water, 20% is protein, and the remaining 5% is substances such as salts, enzymes, minerals, carbohydrates, fats, amino acids, and highenergy phosphates. Myosin, actin, troponin and tropomyosin are the most important proteins.
Skeletal muscles play a vital role in locomotion, heat production, support of soft tissues, and overall metabolism. They have a remarkable ability to adapt to a variety of environmental stimuli, including regular physical training (e.g. endurance or strength exercise) (Aagaard and Andersen, 1998; Andersen et al., 2005; Holm et al., 2008; Parcell et al., 2005), substrate availability (Bohé et al., 2003; Kraemer et al., 2009; Phillips, 2009; Tipton et al., 2009), and unloading conditions (Alkner and Tesch, 2004; Berg, Larsson and Tesch, 1997; Caiozzo et al., 2009; Lemoine et al., 2009; Trappe et al., 2009).
This chapter will describe skeletal muscle’s basic structure and function, contraction mechanism, fibre types and hypertrophy. Its integration with the neural system will be the focus of the next chapter.
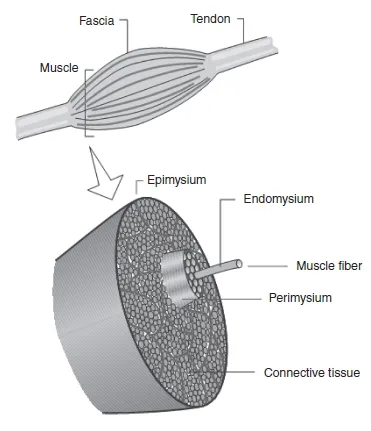
1.1.2 SKELETAL MUSCLE MACROSTRUCTURE
Skeletal muscles are essentially composed of specialized contracting cells organized in a hierarchical fashion supported by a connective tissue framework. The entire muscle is surrounded by a layer of connective tissue called fascia. Underneath the fascia is a thinner layer of connective tissue called epimysium which encloses the whole muscle. Right below is the perimysium, which wraps a bundle of muscle fibres called fascicle (or fasciculus), thus; a muscle is formed by several fasciculi. Lastly, each muscle fibre is covered by a thin sheath of collagenous connective tissue called endomysium (Figure 1.1.1).
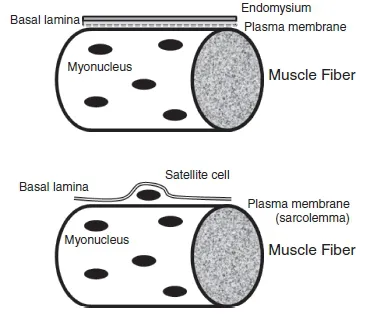
Directly beneath the endomysium lies the sarcolemma, an elastic membrane which contains a plasma membrane and a basement membrane (also called basal lamina). Sometimes, the term sarcolemma is used as a synonym for muscle-cell plasma membrane. Among other functions, the sarcolemma is responsible for conducting the action potential that leads to muscle contraction. Between the plasma membrane and basement membrane the satellite cells are located (Figure 1.1.2). Their regenerative function and possible role in muscle hypertrophy will be discussed later in this chapter.
All these layers of connective tissue maintain the skeletal muscle hierarchical structure and they combine together to form the tendons at each end of the muscle. The tendons attach muscles to the bones and transmit the force they generate to the skeletal system, ultimately producing movement.
1.1.3 SKELETAL MUSCLE MICROSTRUCTURE
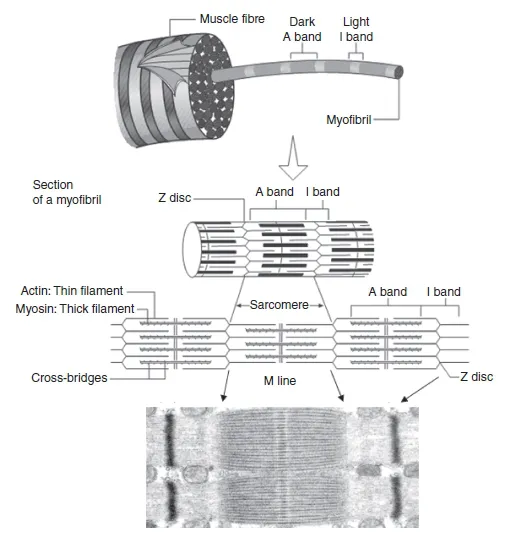
Muscle fibre s, also called muscle cell s or myofibre s, are long, cylindrical cells 1-100µm in diameter and up to 30–40 cm in length. They are made primarily of smaller units called myofibril s which lie in parallel inside the muscle cells (Figure 1.1.3). Myofibrils are contractile structures made of myofilament s named actin and myosin. These two proteins are responsible for muscle contraction and are found organized within sarcomere s (Figure 1.1.3). During skeletal muscle hypertrophy, myofibrils increase in number, enlarging cell size.
A unique characteristic of muscle fibres is that they are multinucleated (i.e. have several nuclei). Unlike most body cells, which are mononucleated, a muscle fibre may have 250–300 myonuclei per millimetre (Brooks, Fahey and Baldwin, 2005; McArdle, Katch and Katch 2007). This is the result of the fusion of several individual mononucleated myoblast s (muscle’s progenitor cells) during the human body’s development. Together they form a myotube, which later differentiates into a myofibre. The plasma membrane of a muscle cell is often called sarcolemma and the sarcoplasm is equivalent to its cytoplasm. Some sarcoplasmic organelles such as the sarcoplasmic reticulum and the transverse tubules are specific to muscle cells.
1.1.3.1 Sarcomere and myofilaments
A sarcomere is the smallest functional unit of a myofibre and is described as a structure limited by two Z disks or Z lines (Figure 1.1.3). Each sarcomere contains two types of myofilament: one thick filament called myosin and one thin filament called actin. The striated appearance of the skeletal muscle is the result of the positioning of myosin and actin filaments inside each sarcomere. A lighter area named the I band is alternated with a darker A band. The absence of filament overlap confers a lighter appearance to the I band, which contains only actin filaments, while overlap of myosin and actin gives a darker appearance to the A band. The Z line divides the I band in two equal halves; thus a sarcomere consists of one A band and two half-I bands, one at each side of the sarcomere. The middle of the A band contains the H zone, which is divided in two by the M line. As with the I band, there is no overlap between thick and thin filaments in the H zone. The M line comprises a number of structural proteins which are responsible for anchoring each myosin filament in the correct position at the centre of the sarcomere.
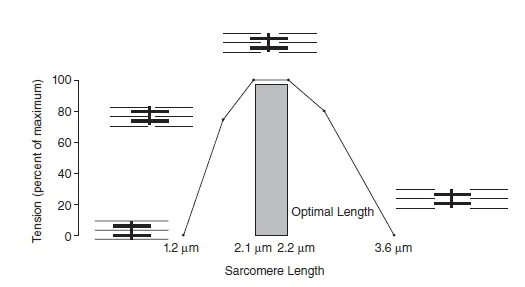
On average, a sarcomere that is between 2.0 and 2.25 µm long shows optimal overlap between myosin and actin filaments, providing ideal conditions for force production. At lengths shorter or larger than this optimum, force production is compromised (Figure 1.1.4).
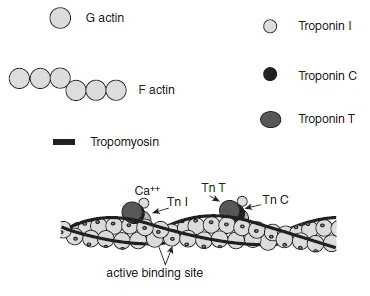
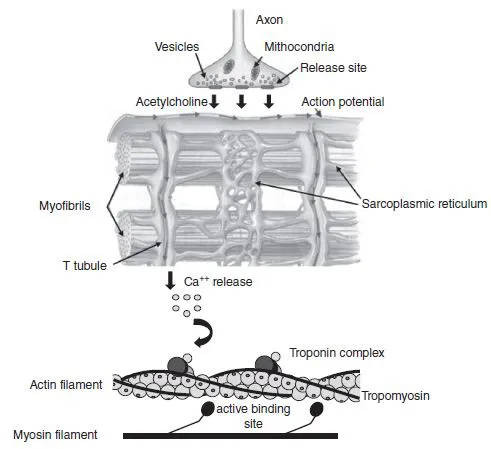
The actin filament is formed by two intertwined strands (i.e. F-actin) of polymerized globular actin monomers (G-actin). This filament extends inward from each Z line toward the centre of the sarcomere. Attached to the actin filament are two regulatory proteins named troponin (Tn) and tropomyosin (Tm) which control the interaction between actin and myosin. Troponin is a protein complex positioned at regular intervals (every seven actin monomers) along the thin filament and plays a vital role in calcium ion (Ca + +) reception. This regulatory protein complex includes three components: troponin C (TnC), which binds Ca + +, troponin T (TnT), which binds tropomyosin, and troponin I (T nI), which is the inhibitory subunit. These subunits are in charge of moving tropomyosin away from the myosin binding site on the actin filament during the contraction process. Tropomyosin is distributed along the length of the actin filament in the groove formed by two F-actin strands and its main function is to inhibit the coupling between actin and myosin filaments from blocking active actin binding sites (Figure 1.1.5).
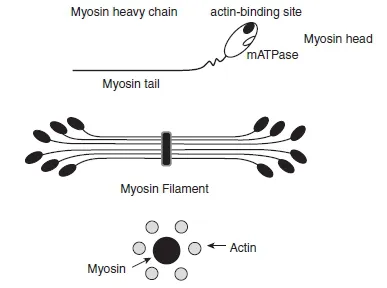
The thick filament is made mostly of myosin protein. A myosin molecule is composed of two heavy chains (MHCs) and two pairs of light chains (MLCs). It is the MHCs that determines muscle fibre phenotype. In mammalian muscles, the MHC component exists in four different isoforms (types I, IIA, IIB, and IIX), whereas the MLCs can be separated into two essential LCs and two regulatory LCs. Myosin heavy and light chains combine to fine tune the interaction between actin and myosin filaments during muscle contraction. A MHC is made of two distinct parts: a head (heavy meromyosin) and a tail (light meromyosin), in a form that may be compared to that of a golf club (Figure 1.1.6).
Approximately 300 myosin molecules are arranged tail-to-tail to constitute each thick filament (Brooks, Fahey and Baldwin, 2005). The myosin heads protrude from the filament; each is arranged in a 60 ° rotation in relation to the preceding one, giving the myosin filament the appearance of a bottle brush. Each myosin head attaches directly to the actin filament and contains ATPase activity (see Section 1.1.5). Actin and myosin filaments are organized in a hexagonal lattice, which means that each myosin filament is surrounded by six actin filaments, providing a perfect match for interaction during contraction (Figure 1.1.6).
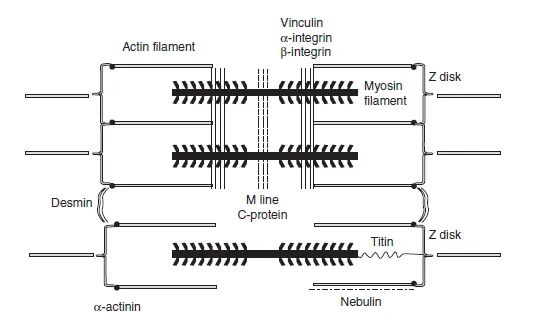
A large number of other proteins can be found in the interior of a muscle fibre and in sarcomeres; these constitute the so-called cytoskeleton. The cytoskeleton is an intracellular system composed of intermediate filaments; its main functions are to (1) provide structural integrity to the myofibre, (2) allow lateral force transmissions among adjacent sarcomeres, and (3) connect myofibrils to the cell’s plasma membrane. The following proteins are involved in fulfilling these functions (Bloch and Gonzalez-Serratos, 2003; Hatze, 2002; Huijing, 1999; Monti et al., 1999): vinculin, α-and β-integrin (responsible for the lateral force transmission within the fibre), dystrophin (links actin filaments with the dystroglycan and sarcoglycan complexes, which are associated with the sarcolemma and provide an extracellular connection to the basal lamina and the endomysium), α-actinin (attaches actin filaments to the Z disk), C protein (maintains the myosin tails in a correct spatial arrangement), nebulin (works as a ruler, assisting the correct assembly of the actin filament), titin (contributes to secure the thick filaments to the Z lines), and desmin (links adjacent Z disks together). Some of these proteins are depicted in Figure 1.1.7.
1.1.3.2 Sarcoplasmic reticulum and transverse tubules
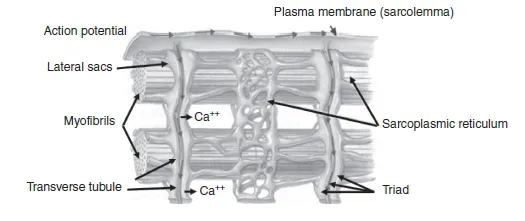
The sarcoplasmic reticulum (SR) of a myofibre is a specialized form of the endoplasmic reticulum found in most human body cells. Its main functions are (1) intracellular storage and (2) release and uptake of Ca + + associated with the regulation of muscle contraction. In fact, the release of Ca + + upon electrical stimulation of the muscle cell plays a major role in excitation–contraction coupling (E-C coupling; see Section 1.1.4). The SR can be divided into two parts: (1) the longitudinal SR and (2) the junctional SR (Rossi and Dirksen, 2006; Rossi et al., 2008). The longitudinal SR, which is responsible for Ca + + storage and uptake, comprises numerous interconnected tubules, forming an intricate network of channels throughout the sarcoplasm and around the myofibrils. At specific cell regions, the ends of the longitudinal tubules fuse into single dilated sacs called terminal cisternae or lateral sacs. The junctional SR is found at the junction between the A and the I bands and represents the region responsible for Ca + + release (Figure 1.1.8).
The transverse tubules (T tubules) are organelles that carry the action potential (electrical stimulation) from the sarcolemma surface to the interior of the muscle fibre. Indeed, they are a continuation of the sarcolemma and run perpendicular to the fibre orientation axis, closely interacting with the SR. The association of a T tubule with two SR lateral sacs forms a structure called a triad which is located near each Z line of a sarcomere (Figure 1.1.8). This arrangement ensures synchronization between the depolarization of the sarcolemma and the release of Ca + + in the intracellular medium. It is the passage of an action potential down the T tubules that causes the release of intracellular Ca + + from the lateral sacs of the SR, causing muscle contraction; this is the mechanism referred to as E-C coupling (Rossi et al., 2008).
1.1.4 CONTRACTION MECHANISM
1.1.4.1 Excitation-contraction (E-C) coupling
A muscle fibre has the ability to transform chemical energy into mechanical work through the cyclic interaction between actin and myosin filaments during contraction. The process starts with the arrival of an action potential and the release of Ca + + from the SR during the E-C coupling. An action potential is delivered to the muscle cell membrane by a motor neuron through the release of a neurotransmitter named acetylcholine (ACh). The release of ACh opens specific ion channels in the muscle plasma membrane, allowing sodium ions to diffuse from the extracellular medium into the cell, leading to the depolarization of the membrane. The depolarization wave spreads along the membrane and is carried to the cell’s interior by the T tubules. As the action potential travels down a T tubule, calcium channels in the lateral sacs of the SR are opened, releasing Ca + + in the intracellular fluid. Calcium ions bind to troponin and, in the presence of ATP, start the process of skeletal muscle contraction (Figure 1.1.9).
1.1.4.2 Skeletal muscle contraction
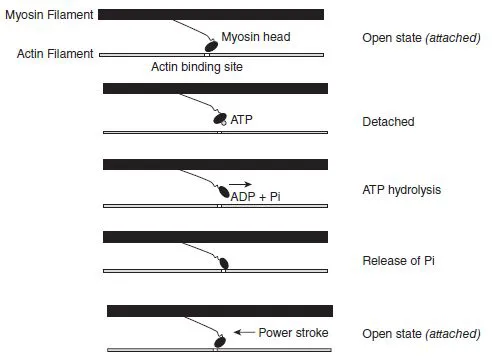
Based on a three-state model for actin activation (McKillop and Geeves, 1993), the thin filament state is influenced by both Ca + + binding to troponin C (TnC) and myosin binding to actin. The position of tropomyosin (Tm) on actin generates a blocked, a closed, or an open state of actin filament activation. When intracellular Ca+ + concentration is low, and thus Ca + + binding to TnC is low, the thin filament stays in a blocked state because Tm position does not allow actin-myosin interaction. However, the release of Ca+ + by the SR and its binding to TnC changes the conformational shape of the troponin complex, shifting the actin filament from a blocked to a closed state. This transition allows a weak interaction of myosin heads with the actin filament, but during the closed state the weak interaction between myosin and actin does not produce force. Nevertheless, the weak interaction induces further movements in Tm, shifting the actin filament from the closed to the open state, resulting in a strong binding of myosin heads. In the open state, a high affinity between the myosin heads and the actin filament provides an opportunity to force production.
The formation of the acto-myosin complex permits the myosin head to hydrolyse a molecule of ATP and the free energy liberated is used to produce mechanical work (muscle contraction) during the cross-bridge power stroke (Vandenboom, 2004). Cross-bridges are projections from the myosin filament in the region of overlap with the actin filament. The release of ATP hydrolysis byproducts (Pi and ADP) induces structural changes in the acto-myosin complex that promote the crossbridge cycle of attachment, force generation, and detachment. The force-generation phase is usually called the ‘power stroke’. During this phase, actin and myosin filaments slide past each other and the thin filaments move toward the centre of the sarcomere, causing its shortening (Figure 1.1.10). Each crossbridge power stroke produces a unitary force of 3–4 pico Newtons (1 pN = 10−12 N) over a working distance of 5 –15 nanometres (1nm = 10 −9 m) against the actin filament ...