
eBook - ePub
Peptide Drug Discovery and Development
Translational Research in Academia and Industry
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Peptide Drug Discovery and Development
Translational Research in Academia and Industry
About this book
Filling a real knowledge gap, this handbook and ready reference is both modern and forward-looking in its emphasis on the "bench to bedside" translational approach to drug development.
Clearly structured into three major parts, the book stakes out the boundaries of peptide drug development in the preclinical as well as clinical stages. The first part provides a general background and focuses on the characteristic strengths and weaknesses of peptide drugs. The second section contains five cases studies of peptides from diverse therapeutic fields, and the lessons to be learned from them, while the final part looks at new targets and opportunities, discussing several drug targets and diseases for which peptide drugs are currently being developed.
Clearly structured into three major parts, the book stakes out the boundaries of peptide drug development in the preclinical as well as clinical stages. The first part provides a general background and focuses on the characteristic strengths and weaknesses of peptide drugs. The second section contains five cases studies of peptides from diverse therapeutic fields, and the lessons to be learned from them, while the final part looks at new targets and opportunities, discussing several drug targets and diseases for which peptide drugs are currently being developed.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Peptide Drug Discovery and Development by Miguel Castanho,Nuno Santos in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Part I
The Academia – Market Bouncing of Peptide Drugs – Challenges and Strategies in Translational Research with Peptide Drugs
Chapter 1
Peptides as Leads for Drug Discovery
1.1 Introduction
Peptides have long been used as a source of active material for drug discovery but their use as marketed medicaments is often limited to intravenous administration. The received opinion for peptide drugs delivered via the oral route is that this is a highly challenging endeavor due to the peptides propensity for proteolytic degradation, high clearance, and resulting problems with its delivery, such as low oral bioavailability; all arising from the inclusion of a number of peptide bonds. Scientists have, therefore, sought peptide mimics (peptidomimetics) [1] where degradation of the peptide bonds is hindered (through, for example, N-methylation of the amide nitrogen) and de-peptidization through morphing of the amide (peptide) bonds into peptoids, for example, or through making the molecule more like a small molecule than a peptide. The peptidomimetic would have similar secondary structure as well as other structural features analogous to that of the original peptide, which allows it to displace the original peptide, or protein, from receptors or enzymes [2]. It is in the context of peptidomimics that this work will focus, on peptide-based discovery that has led to advanced (pre-)clinical candidates which are delivered by the oral route of administration.
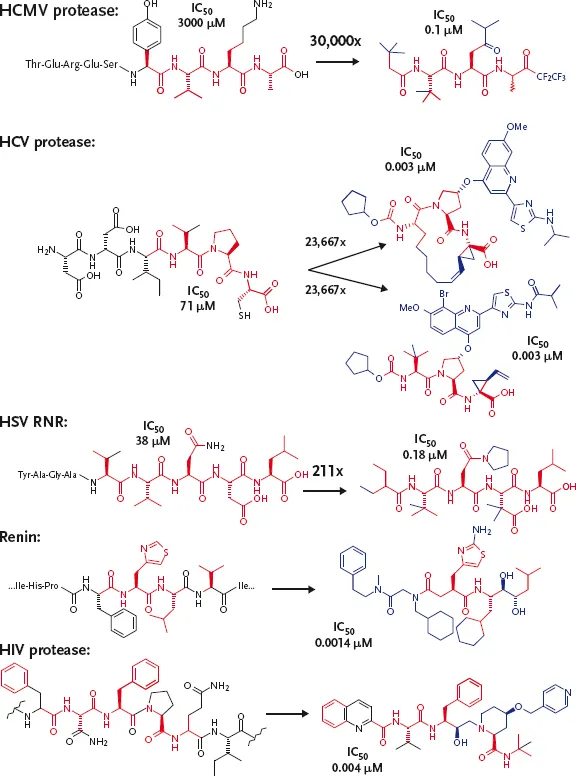
Fortunately, advances in technologies such as phage-display screening, have enabled the high-throughput discovery of peptides that can inhibit a desired biological reaction for drug discovery purposes [3]. It is now also possible to enable the rapid optimization of peptide mimics to satisfy the many hurdles of drug development. This review intends to expose the fascinating properties and handles that peptides offer and how, through “sensemaking” – that is, the process of gathering and interpreting a body of information relevant to a problem [4] – leading to knowledge building within the project, coupled to synthetic and analytical strategies, successful processes may be adopted that can deliver peptide mimetic (pre-) clinical candidates. We will emphasize our experience from in-house programs where peptide leads were successfully advanced to pre-clinical and clinical candidates (see Figure 1.1). Critical lessons, novel strategies, and examples will be explored at various stages of this process, ranging from the discovery of peptide leads to those that have entered clinical trials. Particularly, we propose that our strategy and work flow (see Schemes 1.1 and 1.2) can represent an expeditious way to render peptides to drugs. This review will be based on exposing major findings/lessons that have commonality among systems and that can advance drug discovery rapidly, if used appropriately. Central for this purpose, it is demonstrated in Figure 1.1 that there can be a significant degree of similarity between the original peptide hit and the advanced analog. Figure 1.1 shows that one can conserve major segments and structural features of relatively weak lead peptides that are required for achieving potency in drugs (colored as red in Figure 1.1). Also critical are truncations and alterations (colored black) and the addition of new features (colored in blue). In summary, we believe that if one can exploit the latent structural functionality on the peptide starting points effectively, then delivery of orally bioavailable drugs derived from a peptide lead becomes possible.
Figure 1.1 Structures of peptide starting points (leads) and their advanced peptide mimics (drugs). The conserved bonds/atoms in both the lead and drug are colored red, whereas the blue and black represent new and modified or deleted sections, respectively.
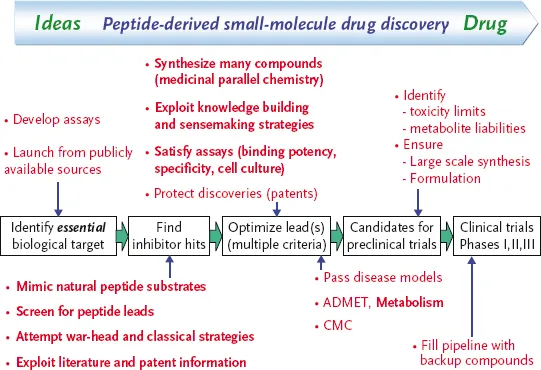
Scheme 1.1 An overview of the peptide-derived, small-molecule drug discovery. Highlighted in bold text are discovery periods where “sensemaking” and knowledge building cycles can be employed during peptide optimization.
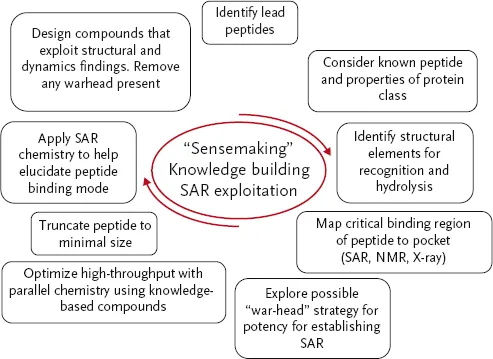
Scheme 1.2 An overview of the drug discovery periods that impact and fuel the “sensemaking,” knowledge building, and SAR exploitation cycles.
1.2 Overview of Process for Transforming Peptides to Peptidomimetics
The pharmaceutical industry has developed complex and fascinating processes for discovering and optimizing leads that become drugs. Scheme 1.1 depicts an overview of how one might progress a peptide to peptidomimetic drug project, indicating the various stages (bold text) that will be exemplified in the projects that follow. Of central importance is the “sensemaking” phase (Scheme 1.2), supported by knowledge building, including mapping of the critical binding parts of the peptide with model creation and peptide truncation, and matching of the free state of the peptide to the bioactive conformation through, for example, rigidification, and de-peptidization.
This chapter will consider a number of case studies against different targets where, after hit identification, the minimal peptide fragments were elucidated and then subjected to conformational rigidification. It is well understood that the binding of a ligand to a macromolecule involves numerous recognition events that are strongly influenced by forces such as van der Waal contacts, electrostatic interactions, solvation effects, and also by ligand to macromolecule shape complementarity. Less well appreciated, but no less important and as critical to these recognition events, are the necessary structural and flexibility adaptations of the ligand and receptor to attain the bioactive complex. Therefore, when considering utilizing a peptide hit as a starting point for drug discovery, the tactics utilized should move beyond the classical “lock-and-key” model to a more holistic approach that incorporates the effects of dynamics and conformational changes. In doing so, rational drug design efforts could be accelerated from the knowledge of these adaptive processes. However, to date, few reports of the application of dynamics and conformational changes have appeared in the literature. In part this is due to the paucity of experimental methods that can provide the type of atomic-level information required. Thus, their importance and impact in drug design have not yet been fully realized.
The process we propose may be summarized (Scheme 1.2) as follows:
- Identify lead peptides.
- Understand properties of the protein.
- Map critical binding elements of substrate peptide.
- Understand (by X-ray/NMR) protein–ligand interactions.
- Increase potency, for example, by using a warhead if necessary, to provide meaningful structure–activity relationships (SAR).
- Truncation to minimally active peptide (allowing for initial losses in potency as needed).
- Elucidate free versus bound conformations and ensure SAR designs produce compounds with free conformations matching the bioactive one.
- In parallel, de-peptidize molecule (e.g., by including bulky side-chains or altering the backbone) and remove any warhead present.
This process will be exemplified through the following examples.
1.3 HCMV Protease
1.3.1 HCMV Protease: Identification and Characterization of Antiviral Inhibitors Targeting the Serine Protease Domain of the Human Cytomegalovirus (HCMV Protease)
Human Cytomegalovirus (HCMV) is a pathogen and member of the herpesvirus family that is highly prevalent in the human population [5]. This virus poses a significant risk to immunocompromized individuals, organ transplant recipients and neonates who acquire the infection congenitally [6, 7]. HCMV encodes a unique protease involved in capsid assembly and this protease enzyme is responsible for processing the assembly protein; the latter protein’s function is analogous to that of the “scaffolding” protein of bacteriophages [8] and its activity is essential to the production of infectious virions [9–12].
The full-length HCMV protease precursor contains 708 amino acids encoded by the UL80 gene. It was discovered that the enzyme can process its own C-terminus and that the protease can also undergo self-processing at the release site near its amino terminus. This cleavage liberates the 256 amino acid catalytic domain, or HCMV protease. Although this enzyme belongs to the serine protease family, differences between familial members exist, as evidenced through X-ray crystallographic analyses [13–16]. These analyses have shown that it possesses a unique protein fold and an unusual catalytic triad (a histidine replacing the more common aspartate). Additionally its activity arises exclusively from its dimer form [17, 18]. Spectroscopic studies [19] have demonstrated that the binding of substrate-based competitive inhibitors results in a conformational change in the enzyme and that catalysis by HCMV protease is performed through an “induced fit” model [20, 21]. Faced with a need to develop a potent HCMV protease inhibitor, the following research process was undertaken.
1.3.2 Mapping Essential Elements of the Substrate Peptides and Determining Structures of Ligands Bound to HCMV
As substrate hydrolysis by HCMV protease was essential for viral capsid assembly, the first task was to decipher the minimal structural elements of the substrates that were required for recognition and hydrolysis. Enzymological studies revealed that peptides which corresponded to 17 amino acids of the release- and maturation-sites (R-site and M-site peptides) were sufficient to induce hydroly...
Table of contents
- Cover
- Contents
- Title
- Copyright
- Preface
- List of Contributors
- Part I: The Academia – Market Bouncing of Peptide Drugs – Challenges and Strategies in Translational Research with Peptide Drugs
- Part II: Peptide Drugs’ Translational Tales – Peptide Drugs Before, Through and After Industry Pipelines
- Part III: Whither Peptide Drugs? Peptides Shaping the Future of Drug Development
- Index