
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
In-situ Synthesis of Polymer Nanocomposites
About this book
The book series "Polymer Nano-, Micro- and Macrocomposites" provides complete and comprehensive information on all important
aspects of polymer composite research and development, including, but not limited to synthesis, filler modification, modeling, characterization as well as application and commercialization issues. Each book focuses on a particular topic and gives a balanced in-depth overview of the respective subfi eld of polymer composite science and its relation to industrial applications. With the books the readers obtain dedicated resources with information relevant to their research, thereby helping to save time and money.
In-situ intercalative polymerization in the presence of filler provides distinct advantages when compared to other nanocomposite synthesis
techniques including the possibility to polymerize a large range of thermoplastic and thermosetting polymers, improved handling of gaseous
or liquid monomers or high pressure polymerization and improved control of heat of polymerization. This volume aims to highlight these
advantages of the generation of polymer nanocomposites with a large spectrum of polymer matrices. Following an overview of the synthesis
methodologies, the text goes on to discuss the most relevant polymer materials, including polyamides, polyolefi nes, polyacrylates, polyethylenes, polyurethanes, polyesters and polyepoxides.
aspects of polymer composite research and development, including, but not limited to synthesis, filler modification, modeling, characterization as well as application and commercialization issues. Each book focuses on a particular topic and gives a balanced in-depth overview of the respective subfi eld of polymer composite science and its relation to industrial applications. With the books the readers obtain dedicated resources with information relevant to their research, thereby helping to save time and money.
In-situ intercalative polymerization in the presence of filler provides distinct advantages when compared to other nanocomposite synthesis
techniques including the possibility to polymerize a large range of thermoplastic and thermosetting polymers, improved handling of gaseous
or liquid monomers or high pressure polymerization and improved control of heat of polymerization. This volume aims to highlight these
advantages of the generation of polymer nanocomposites with a large spectrum of polymer matrices. Following an overview of the synthesis
methodologies, the text goes on to discuss the most relevant polymer materials, including polyamides, polyolefi nes, polyacrylates, polyethylenes, polyurethanes, polyesters and polyepoxides.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access In-situ Synthesis of Polymer Nanocomposites by Vikas Mittal in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Materials Science. We have over one million books available in our catalogue for you to explore.
Information
1
In-situ Synthesis of Polymer Nanocomposites
1.1 Introduction
It was the pioneering work of Toyota researchers toward the development of polymeric nanocomposites in the early 90s [1, 2], in which electrostatically held 1-nm-thick layers of the layered aluminosilicates were dispersed in the polyamide matrix on a nanometer level, which led to an exponential growth in the research in these layered silicate nanocomposites. These nanocomposites were based on the in-situ synthesis approach in which monomer or monomer solution was used to swell the filler interlayers followed by polymerization. Subsequently, Giannelis and coworkers [3, 4] also reported the route of melt intercalation for the synthesis of polymer nanocomposites.
Montmorillonite has been the most commonly used layered aluminosilicate in most of the studies on polymer nanocomposites. The general formula of montmorillonites is Mx(Al4−xMgx)Si8O20(OH)4 [5, 6]. Its particles consist of stacks of 1-nm-thick aluminosilicate layers (or platelets) with a regular gap in between (interlayer). Each layer consists of a central Al-octahedral sheet fused to two tetrahedral silicon sheets. In the tetrahedral sheets, silicon is surrounded by four oxygen atoms, whereas in the octahedral sheets, aluminum atom is surrounded by eight oxygen atoms. Isomorphic substitutions of aluminum by magnesium in the octahedral sheet generate negative charges, which are compensated for by alkaline-earth- or hydrated alkali-metal cations. Owing to the low charge density (0.25–0.5 equiv. mol−1) of montmorillonites, a larger area per cation is available on the surface that leads to a lower interlayer spacing in the modified montmorillonite after surface ion exchange with alkyl ammonium ions. On the contrary, the minerals with high charge density (1 equiv. mol−1) like mica have much smaller area per cation and can lead to much higher basal plane spacing after surface modification; however, owing to very strong electrostatic forces present in the interlayers due to the increased number of ions, these minerals do not swell in water and thus do not allow the cation exchange. In contrast, aluminosilicates with medium charge densities of 0.5–0.8 equiv. mol−1 like vermiculite offer a potential of partial swelling in water and cation exchange that can lead to much higher basal plane spacing in the modified mineral if optimum ion exchange is achieved. Vaia et al. [7] also proposed further insight into the positioning of the surface modification molecules on the surface of the filler based on FTIR experiments. By monitoring frequency shifts of the asymmetric CH2 stretching and bending vibrations, they found that the intercalated chains exist in states with varying degrees of order. In general, as the interlayer packing density or the chain length decreases (or the temperature increases), the intercalated chains adopt a more disordered, liquid-like structure resulting from an increase in the gauche/trans conformer ratio.
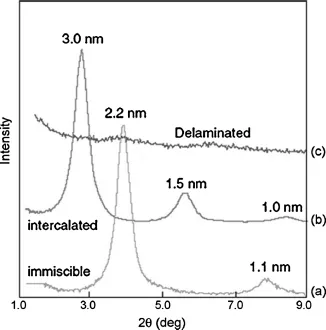
Nanocomposites with a large number of polymer matrices have been synthesized and significant enhancements in the composite properties have been reported. The improvement in the mechanical properties of the nanocomposites is generally reported, though a synergistic enhancement in the other composite properties like gas barrier resistance is also generally achieved. Figure 1.1a demonstrates the decrease in oxygen permeation through the polyurethane, epoxy, and polypropylene nanocomposites as a function of inorganic filler volume fraction [8–10]. Figure 1.1b also shows the improvement in mechanical properties of the polypropylene and polyethylene nanocomposites as a function of filler volume fraction [11–13]. The polypropylene composites have been generated by using two different filler surface modifications containing ammonium and imidazolium ions. The microstructure of the nanocomposites is also ideally classified as unintercalated (phase separated), intercalated, and exfoliated composites. The composite microstructure is classified as exfoliated when the filler platelets are completely delaminated into their primary nanometer scale size and the platelets are far apart from each other so that the periodicity of this platelet arrangement is totally lost. When a single or sometimes more than one extended polymer chain is intercalated into the clay interlayers, but the periodicity of the clay platelets is still intact, such a microstructure is termed as intercalated. On the basis of the interfacial interactions and mode of mixing of the organic and inorganic phases, it is possible that both the phases do not intermix at all and a microcomposite or unintercalated composite is formed. Transmission electron microscopy (TEM) and X-ray diffraction (XRD) are the most commonly used methods to characterize the microstructure of the nanocomposites. Figure 1.2 shows the TEM micrographs depicting the various idealized morphologies of the polymer nanocomposite structures [15]. However, it should be noticed that these classifications of the composite microstructure as exfoliated and intercalated are not very realistic as generally in reality a mixture of different morphologies is present. Figure 1.3 also shows the three idealized morphologies of immiscible, intercalated, and exfoliated composites [16]. The presence or absence of diffraction peaks in the XRD of the composites is used to assess information about the microstructure of the composites. The intensity of the X-ray diffractograms is generally taken as a measure to classify the microstructure as intercalated or exfoliated. However, it should be noticed that the X-ray signal are very qualitative in nature and are strongly influenced by the sample preparation, orientation of the platelets, as well as defects present in the crystal structure of the montmorillonites. Therefore, the classification of the nanocomposite microstructure just based on the intensity can be faulty. Also, the presence of diffraction signal in the diffractograms of the composite does not mean that 100% of the microstructure is intercalated and it is quite possible to have significant amount of exfoliation present in the composite. Similarly, absence of diffraction signal also does not guarantee the complete exfoliation as small or randomly oriented intercalated platelets may still be present in the composite.
Figure 1.1 (a) Relative oxygen permeation and (b) relative tensile modulus of various polymer nanocomposites as a function of filler volume fraction [8–13].
Reproduced from Ref. [14].
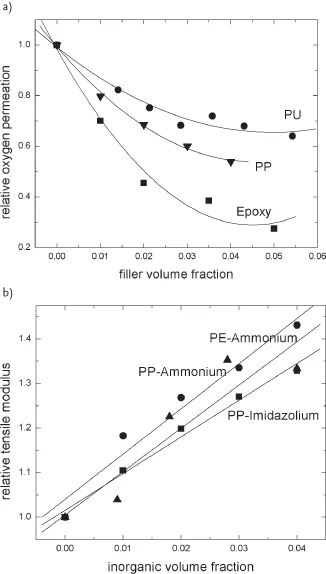
Figure 1.2 TEM micrographs indicating various possible morphologies in the composites as a function of the filler distribution: (a) exfoliated, (b) intercalated, and (c) unintercalated.
Reproduced from Ref. [15] with permission from Wiley.
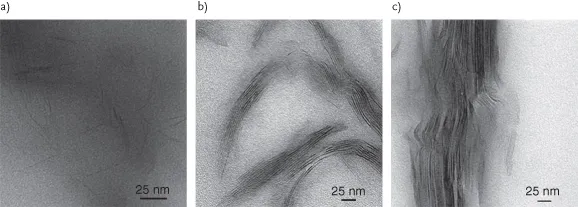
Figure 1.3 XRD patterns of immiscible, intercalated, and exfoliated composites.
Reproduced from Ref. [16] with permission from Elsevier.
Many factors influence the microstructure and hence the properties of the nanocomposites. The first of such factors is the surface modification of the filler and its interaction with the polymer. The modification is required to make the filler organophilic and to push the filler interlayers apart, thus providing possibilities for polymer intercalation. Table 1.1 details the various kinds of surface modifications commonly used to modify the filler surface. The basal plane spacing of the filler resulting after the surface modification is also provided. The modifications differ in many aspects such as chain length, density of the chains in the surface modification molecule, and chemical architecture. The modification molecules have specific interactions with the matrix polymer and these interactions are responsible for the ability of the filler to exfoliate or delaminate in the polymer matrix [8, 9]. Figure 1.4 shows an exampl...
Table of contents
- Cover
- Series page
- Title page
- Copyright page
- Preface
- List of Contributors
- 1 In-situ Synthesis of Polymer Nanocomposites
- 2 Polyamide Nanocomposites by In-situ Polymerization
- 3 Polyolefin–Clay Nanocomposites by In-situ Polymerization
- 4 Gas-Phase-Assisted Surface Polymerization and Thereby Preparation of Polymer Nanocomposites
- 5 PET Clay Nanocomposites by In-situ Polymerization
- 6 Control of Filler Phase Dispersion in Bio-Based Nanocomposites by In-situ Reactive Polymerization
- 7 Polyurethane Nanocomposites by In-situ Polymerization Approach and Their Properties
- 8 In-situ Synthesis and Properties of Epoxy Nanocomposites
- 9 Unsaturated Polyester–Montmorillonite Nanocomposites by In-situ Polymerization
- 10 Polymer Clay Nanocomposites by In-situ Atom Transfer Radical Polymerization
- 11 Polybutadiene Clay Nanocomposites by In-situ Polymerization
- 12 P3HT–MWNT Nanocomposites by In-situ Polymerization and Their Properties
- 13 Polystyrene–Montmorillonite Nanocomposites by In-situ Polymerization and Their Properties
- 14 Aliphatic Polyester and Poly(ester amide) Clay Nanocomposites by In-situ Polymerization
- Index