
- English
- ePUB (mobile friendly)
- Available on iOS & Android
The Physics of Microdroplets
About this book
The Physics of Microdroplets gives the reader the theoretical and numerical tools to understand, explain, calculate, and predict the often nonintuitive observed behavior of droplets in microsystems.
Microdrops and interfaces are now a common feature in most fluidic microsystems, from biology, to biotechnology, materials science, 3D-microelectronics, optofluidics, and mechatronics. On the other hand, the behavior of droplets and interfaces in today's microsystems is complicated and involves complex 3D geometrical considerations. From a numerical standpoint, the treatment of interfaces separating different immiscible phases is difficult.
After a chapter dedicated to the general theory of wetting, this practical book successively details:
- The theory of 3D liquid interfaces
- The formulas for volume and surface of sessile and pancake droplets
- The behavior of sessile droplets
- The behavior of droplets between tapered plates and in wedges
- The behavior of droplets in microchannels
- The effect of capillarity with the analysis of capillary rise
- The onset of spontaneous capillary flow in open microfluidic systems
- The interaction between droplets, like engulfment
-
The theory and application of electrowetting
- The state of the art for the approach of 3D-microelectronics using capillary alignment
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Fundamentals of Capillarity
1.1 Abstract
1.2 Interfaces and Surface Tension
1.2.1 The Notion of Interface
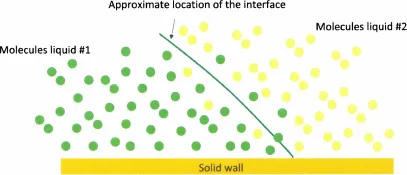

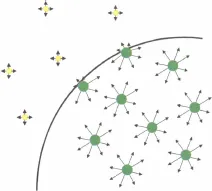
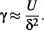
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Acknowledgements
- Preface
- Introduction
- Chapter 1: Fundamentals of Capillarity
- Chapter 2: Minimal Energy and Stability Rubrics
- Chapter 3: Droplets: Shape, Surface and Volume
- Chapter 4: Sessile Droplets
- Chapter 5: Droplets Between Two Non-parallel Planes: From Tapered Planes to Wedges
- Chapter 6: Microdrops in Microchannels and Microchambers
- Chapter 7: Capillary Effects: Capillary Rise, Capillary Pumping, and Capillary Valve
- Chapter 8: Open Microfluidics
- Chapter 9: Droplets, Particles and Interfaces
- Chapter 10: Digital Microfluidics
- Chapter 11: Capillary Self-assembly for 3D Microelectronics
- Chapter 12: Epilogue
- Index