
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Models for Bonding in Chemistry
About this book
A readable little book assisting the student in understanding, in a nonmathematical way, the essentials of the different bonds occurring in chemistry. Starting with a short, self-contained, introduction, Chapter 1 presents the essential elements of the variation approach to either total or second-order molecular energies, the system of atomic units (au) necessary to simplify all mathematical expressions, and an introductory description of the electron distribution in molecules. Using mostly 2x2 Hückel secular equations, Chapter 2, by far the largest part of the book because of the many implications of the chemical bond, introduces a model of bonding in homonuclear and heteronuclear diatomics, multiple and delocalized bonds in hydrocarbons, and the stereochemistry of chemical bonds in polyatomic molecules, in a word, a model of the strong first-order interactions originating the chemical bond. In Chapter 3 the Hückel model of the linear polyene chain is used to explain the origin of band structure in the 1-dimensional crystal. Chapter 4 deals with a simple two-state model of weak interactions, introducing the reader to understand second-order electric properties of molecules and VdW bonding between closed shells. Lastly, Chapter 5 studies the structure of H-bonded dimers and the nature of the hydrogen bond, which has a strength intermediate between a VdW bond and a weak chemical bond. Besides a qualitative MO approach based on HOMO-LUMO charge transfer from an electron donor to an electron acceptor molecule, a quantitative electrostatic approach is presented yielding an electrostatic model working even at its simplest pictorial level. A list of alphabetically ordered references, author and subject indices complete the book.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
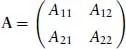
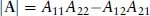
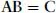
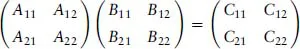
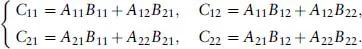
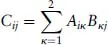
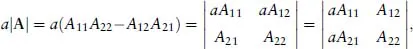
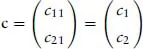
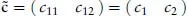
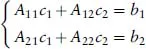
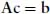
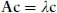
Table of contents
- Cover
- Title page
- Copyright
- Dedication
- Preface
- 1: Mathematical Foundations
- Part 1: Short-range Interactions
- Part 2: Long-Range Interactions
- References
- Author Index
- Subject Index