1.1 Introduction
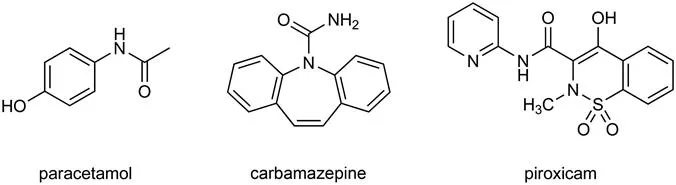
This book is about pharmaceutical crystals. The focus is on small-molecule active pharmaceutical ingredients (APIs) rather than polymers or macromolecules such as proteins. Typical small-molecule APIs are paracetamol, carbamazepine and piroxicam (Figure 1.1). An effective definition of a crystal is that it exhibits long-range order in three dimensions. This means that the spatial arrangement of the molecules repeats regularly in three dimensions on a scale that is much larger than the basic repeating unit of the structure. Amorphous solids, which do not show long-range order, are also important for pharmaceuticals but it is much harder to discuss their structure in any general way.
Figure 1.1 Typical active pharmaceutical ingredients (APIs) considered in this book.
This chapter sets the scene by providing some definitions and classifications. The need to make such classifications is largely practical. We need to know what it means when someone talks about a polymorph or a pharmaceutical co-crystal, and we need to have some appreciation of the implied structure if we are to understand how it is likely to influence the solid-state properties. Some of these definitions may have differing viewpoints expressed in regulatory documents compared to the academic literature. The aim in this chapter is to give a scientific overview, without intending to be definitive and without dwelling too much on legal/regulatory definitions.
1.2 Classifying Pharmaceutical Solids: An Overview
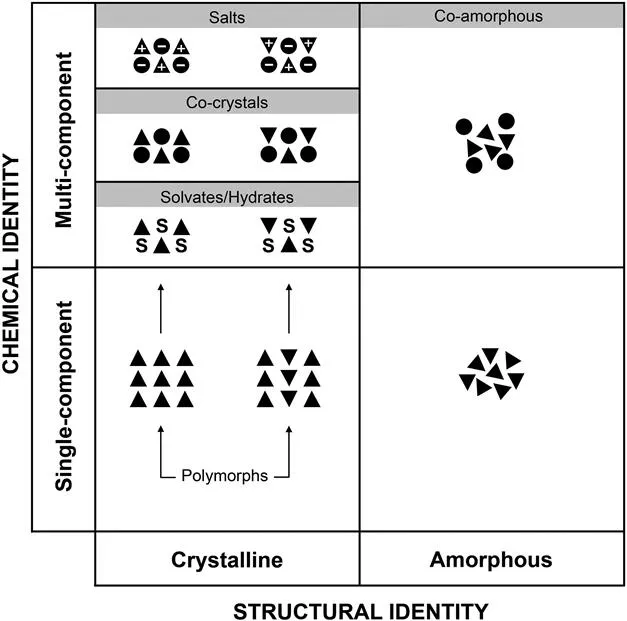
A systematic scheme to classify pharmaceutical solids is suggested in Figure 1.2. Two principal classifications are defined, indicated by the two axes of the diagram. The horizontal axis is associated with structural identity while the vertical axis is associated with chemical identity. The chemical identity refers to the types of molecules that are present. Assuming it is possible to define molecules unambiguously (which it usually is), it is straightforward to say whether a given solid contains one type of molecule or more than one type of molecule. This provides a clear chemical classification: a pharmaceutical solid is either single-component (containing one type of molecule), or multi-component (containing more than one type of molecule). Single-component solids are pure chemical compounds which do not need any further chemical classification. Multi-component solids, however, are commonly divided into numerous chemical sub-classes. Three classes commonly defined for pharmaceutical solids are salts, co-crystals and solvates/hydrates. These classifications will be discussed in more detail in Section 1.4, but for now we will just accept that they exist and describe what they mean.
Figure 1.2 An outline classification of pharmaceutical solids. Key: ▲=API molecule; ●=co-crystal/salt former molecule; S=solvent molecule.
On the horizontal axis of Figure 1.2, the structural identity refers to whether the solid is crystalline or amorphous. A crystalline solid exhibits long-range order in three dimensions, while an amorphous solid does not. The definitions of “crystalline” and “amorphous” will be discussed in Section 1.3; for now, we state that a solid must be either crystalline (being perfectly ordered in three dimensions) or amorphous, and draw a hard boundary between them.
The two-dimensional nature of Figure 1.2 highlights that the different structural classifications can be applied to each of the chemical categories. So, any given chemical composition might potentially exist in both crystalline and amorphous forms. Different crystalline forms with the same chemical composition are called polymorphs. Both single- and multi-component solids could be polymorphic. A given API (such as those in Figure 1.1) might exhibit numerous crystalline polymorphs and an amorphous form; it might be combined with a wide variety of other molecules to give a range of solvates, co-crystals and salts; each multi-component solid might be polymorphic or amorphous, and so on. The wide variety of solid forms that might be encountered for a particular API is sometimes referred to as its solid-form landscape.
1.3 Structural Classification: Crystalline and Amorphous Solids
A crystalline solid exhibits long-range order. An amorphous solid does not exhibit long-range order.
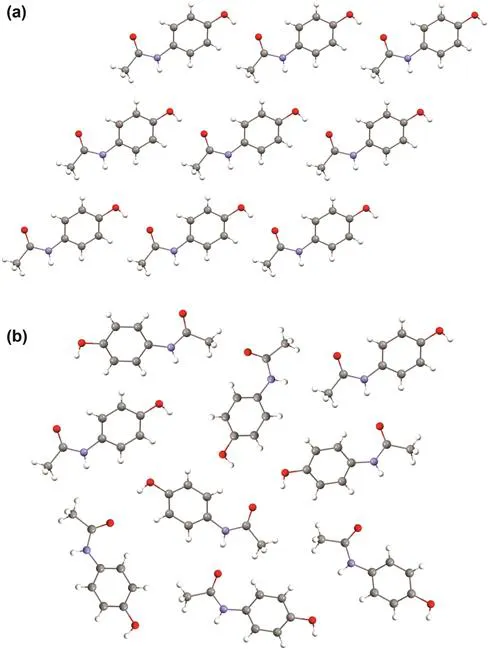
According to the definitions above, the key feature that distinguishes crystalline and amorphous solids is long-range order. An ordered arrangement is one where the positions and orientations of the molecules exhibit a regular repeating pattern, more precisely a periodic pattern, through the entire solid. Thus, the arrangement of the molecules in one small part of the solid is reproduced regularly throughout the entire solid. This does not necessarily mean that all molecules in the solid have exactly the same environment, but usually there will be only a small number of different molecular environments. Chapters 2–4 relate crystalline order specifically to the concept of symmetry. At this stage, it is sufficient to note that Figure 1.3(a) shows an ordered (crystalline) arrangement of paracetamol molecules, while Figure 1.3(b) shows a less ordered (amorphous) arrangement. It should be immediately clear from these diagrams that an absolute distinction cannot be made between crystalline and amorphous. It is not the case that a solid must be either fully ordered or not at all ordered. Instead, we should consider the degree of order, and we should view crystalline and amorphous solids as the end points of a structural continuum.
Figure 1.3 Schematic solid-state arrangements of paracetamol molecules: (a) an ordered (crystalline) arrangement; (b) a less ordered (amorphous) arrangement.
1.3.1 Polymorphism
Polymorphs are crystalline solids with the same chemical composition but different crystal structures.
This definition states explicitly that polymorphism refers to the crystalline state, and that polymorphs must have the same chemical composition. The specification of a crystalline solid distinguishes polymorphs from amorphous forms, while the chemical specification means that a multi-component solid, for example an API hydrate, is not a polymorph of the corresponding API. In principle, the word polymorph should only be applied to a crystalline solid if it is established that different crystal structures exist for the same chemical composition. In other words, polymorphs are sets of crystal structures. Typically, polymorphs are labelled using Roman numerals (form I, form II, etc.) or Greek letters (form α, form β, etc.). However, the notation of polymorphs in the pharmaceutical literature is highly inconsistent and there is no standard practice. Indeed, it is quite common for the same polymorph of a given API to be given different names by different authors.
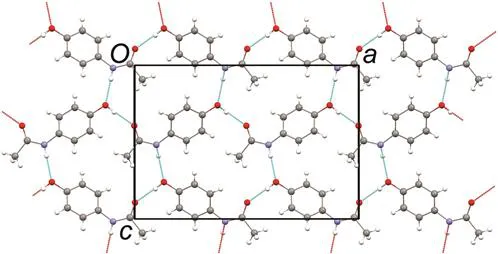
All three APIs in Figure 1.1 are known to be polymorphic. For paracetamol, crystal structures have been determined for three polymorphs to date: two by single-crystal X-ray diffraction and one from powder X-ray diffraction data. The molecular conformations are closely comparable and the primary intermolecular interactions are essentially the same in all three polymorphs: the NH group of the amide forms a hydrogen bond to the O atom of the hydroxyl group, which in turn forms a hydrogen bond to the O atom of a neighbouring amide (Figure 1.4). In two polymorphs (forms II and III), these interactions link the molecules into identical layers, with the distinction between the polymorphs arising mainly from the way in which the layers are stacked on top of each other. By contrast, the molecules in form I adopt layers with a more “corrugated” arrangement (Figure 1.5). This structural difference has a significant influence on the compression properties of the polymorphs, with form I being less susceptible to plastic deformation.
Figure 1.4 Hydrogen-bonded layers within paracetamol form I.
Figure 1.5 Polymorphs of paracetamol: (a) form I, containing corrugated hydrogen-bonded layers; (b) form II, containing flat layers. Form I is less susceptible to plastic deformation.
1.3.2 Polyamorphism
The concept of “polyamorphism” is encountered in the pharmaceutical literature, but it is much less clear than its crystalline counterpart, polymorphism. Principally, this is because polymorphism is a structural concept, and the structures of amorphous solids are inherently less well defined than those of crystalline solids. For crystalline solids, it is possible to define the structure of the entire solid clearly and unambiguously, as we will see in Chapters 2–4, and therefore to specify whether two crystal structures are the same or different. For amorphous solids, it is questionable whether we can define the structure at all. Since the defining feature of an amorphous solid is that it lacks long-range order, the structure in one part of the solid cannot be representative of the whole solid. One feature of an amorphous solid that might be representative is its local structure. This means that there could be some consistency in the way that neighbouring molecules are arranged relative to one another. For example, molecules might be consistently arranged into pairs through some specific intermolecular interaction but the molecular pairs are arranged in a disordered (amorphous) fashion. Alternatively, the molecules could arrange themselves into higher-dimensional motifs such as chains or sheets, but these motifs could extend over only a limited range and/or be aligned in some irregular way. Assuming that local motifs could be defined clearly, polyamorphism might describe amorphous structures with different types or degrees of local order. Again, however, this is problematic because we would have to define what is meant by “local”, and it is in any case practically much more difficult to determine structural information for an amorphous solid. Thus, polyamorphism is a poorly-defined and largely conceptual counterpart to the well-defined polymorphism of crystalline solids. This book focuses on crystalline materials.
1.4 Chemical Classification: Single- and Multi-component Solids
Single-component pharmaceutical solids contain only molecules of one particular API. Multi-component pharmaceutical solids contain molecules of an API plus some other chemical component(s). The additional components may be molecules or single-atom ions such as Na+ or Cl−. Commonly, multi-component pharmaceutical solids are grouped into several sub-classes, based on their chemical composition. The following sections provide some working definitions for three chemical classes that are commonly defined: salts, co-crystals and solvates/hydrates. As stated in Section 1.1, these classifications arise largely from practical needs, and they may become ambiguous at the borderlines. A flexible and coherent outlook is therefore encouraged.
1.4.1 Salts
A salt is a multi-component crystalline solid containing charged molecules and/or ions in a stoichiometric ratio.
The “stoichiometric ratio” specified in this definition is a physical necessity within the solid because any positive charge must be balanced by an equal negative charge. As such, there may be no need for it to be stated explicitly. However, including it provides neat consistency with the definition of a co-crystal given in Section 1.4.2, which is helpful to encourage a coherent view of a salt/co-crystal continuum (...