
Advanced Textbook on Gene Transfer, Gene Therapy and Genetic Pharmacology
Principles, Delivery and Pharmacological and Biomedical Applications of Nucleotide-Based Therapies
- 636 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Advanced Textbook on Gene Transfer, Gene Therapy and Genetic Pharmacology
Principles, Delivery and Pharmacological and Biomedical Applications of Nucleotide-Based Therapies
About this book
This unique advanced textbook provides a clear and comprehensive overview of gene delivery, gene therapy and genetic pharmacology, with descriptions of the main gene transfer vectors and a set of selected therapeutic applications, along with safety considerations. The second edition features new groundbreaking material on genome editing using the recently discovered CRISPR/Cas9 system and on cancer immunotherapy by CAR-T cells. It also presents the historical milestone of gene therapy application in the field of severe combined immunodeficiency, and other fields of gene therapy and molecular medicine.
The use of gene transfer is exponentially growing in the scientific and medical communities for day-to-day cell biology experiments and swift development of gene therapy, which is already revolutionizing medicine. In this advanced textbook, more than 30 leading scientists come together to explore these topics.
This educational introduction provides the background material needed to further explore the subject as well as relevant research literature. It is an invaluable resource to Master, PhD or MD students, post-doctoral scientists or medical doctors, as well as any scientist wishing to deliver a gene or synthetic nucleotide or develop a gene therapy strategy. The second edition's simple and synthetic content will be of value to any reader interested in the biological and medical revolution derived from the elucidation of the human genome.
Contents:
- Basic Definitions and Principles:
- Introduction (Thierry VandenDriessche)
- Basic Definitions and General Principles (Daniel Scherman)
- History of Gene Therapy (Serge Braun)
- Genetic Pharmacology Using Synthetic Deoxyribonucleotides (Jean-Christophe François and Carine Giovannangeli)
- Principles of RNAi Trigger Expression for Gene Therapy (Lisa J Scherer and John J Rossi)
- On Demand Alternative Splicing for Gene Rescue (Stéphanie Lorain and Luis Garcia)
- Nuclease-Mediated Targeted Genetic Correction (Dieter C Gruenert, Hamid Emamekhoo and R Geoffrey Sargent)
- Genome Engineering and Genome Editing Using CRISPR/Cas9–RNA-Guided Nuclease (Daniel Scherman and Jean-Louis Mandel)
- Vectors and Gene Delivery Techniques:
- γ-Retrovirus- and Lentivirus-Derived Vectors for Gene Transfer and Therapy (Caroline Duros and Odile Cohen-Haguenauer)
- Adenovirus Vectors (Stefan Kochanek)
- Adeno-Associated Virus (AAV) Vectors (Aurélie Ploquin, Hildegard Büning and Anna Salvetti)
- Herpes Simplex Virus (HSV-1)-Based Vectors: Applications for Gene Transfer, Gene Therapy, Cancer Virotherapy and Vaccination (Matias E Melendez, Aldo Pourchet, Anna Greco and Alberto L Epstein)
- Non-Viral DNA Vectors (Martin Schleef)
- Macromolecular Conjugates for Non-Viral Nucleic Acid Delivery (Mark Ericson, Kevin Rice and Guy Zuber)
- Auto-Associative Lipid-Based Systems for Non-Viral Nucleic Acid Delivery (Virginie Escriou, Nathalie Mignet and Andrew Miller)
- Hydrodynamic-Pressure-Based Non-Viral Nucleic Acid Delivery (Takeshi Suda, Kenya Kamimura, Guisheng Zhang and Dexi Liu)
- Electrotransfer/Electroporation for Non-Viral Nucleic Acid Delivery (Pascal Bigey, Richard Heller and Daniel Scherman)
- Imaging of Gene Delivery (Georges Vassaux, Peggy Richard-Fiardo, Béatrice Cambien and Philippe Franken)
- Therapeutic Applications:
- Oncolytic Adenoviruses for Cancer Gene Therapy (Gunnel Hallden, Yaohe Wang, Han-Hsi Wong and Nick R Lemoine)
- Progress in DNA Vaccine Approaches for Cancer Immunotherapy (Geoffrey D Hannigan and David B Weiner)
- Adoptive Immunotherapy and CAR-T Cells: A Revolutionary Cell/Gene Therapy to Treat Cancer (Daniel Scherman)
- Gene Therapy for Severe Combined Immunodeficiencies (SCID) (Salima Hacein-Bey-Abina, Alain Fischer and Marina Cavazzana)
- Gene Therapy of the β-Hemoglobinopathies (Emmanuel Payen, Charlotte Colomb, Olivier Negre, Marina Cavazzana Calvo, Salima Hacein-Bey Abina, Yves Beuzard and Philippe Leboulch)
- Gene Therapy for Hemophilia A and B (Nisha Nair, Marinee Chuah and Thierry VandenDriessche)
- Experimental and Clinical Ocular Gene Therapy (Alexis-Pierre Bemelmans and José-Alain Sahel)
- Gene Therapy of Neurological Diseases (Lisa M Stanek, Lamya S Shihabuddin and Seng H Cheng)
- Genetic Therapy of Muscle Diseases: Duchenne Muscular Dystrophy (Takis Athanasopoulos, Susan Jarmin, Helen Foster, Keith Foster, Jagjeet Kang, Taeyoung Koo, Alberto Malerba, Linda Popplewell, Daniel Scherman and George Dickson)
- New Genetic Approaches to Treating Cystic Fibrosis (Stephen L Hart, Amy Walker and Patrick T Harrison)
- Induced Pluripotent Stem Cells and Gene Targeting for Regenerative Medicine (Jizhong Zou and Linzhao Cheng)
- Gene Vector Production:
- Production and Purification of Viral Vectors and Safety Considerations Related to Their Use (Otto-Wilhelm Merten, Matthias Schweizer, Parminder Chahal and Amine Kamen)
- Production and Purification of Plasmid Vectors (Martin Schleef)
Readership: Master, PhD or MD students, post-doctoral scientists or medical doctors, and any scientists using gene transfer techniques or implementing gene therapy strategies.Gene Transfer;Gene Therapy;Gene Delivery;Genetic Pharmacology;Genetic Diseases;Vectors;Nucleotide;Therapy00
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
PART I
BASIC DEFINITIONS AND PRINCIPLES
1
INTRODUCTION
Email: [email protected]
2
BASIC DEFINITIONS AND GENERAL PRINCIPLES
2.1Definitions
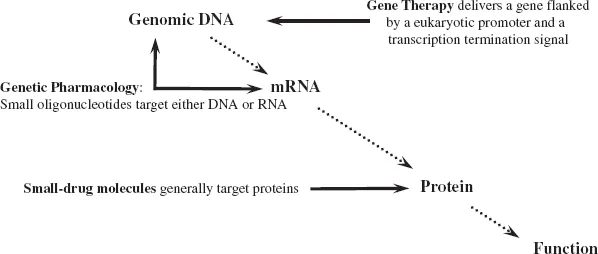

Table of contents
- Cover
- Halftitle
- Series Editors
- Title
- Copyright
- Dedication
- Preface to the First Edition
- Preface to the Second Edition
- About the Editor
- Contents
- Part I: Basic Definitions and Principles
- Part II: Vectors and Gene Delivery Techniques
- Part III: Therapeutic Applications
- Part IV: Gene Vector Production
- Index