
- 288 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Theory of Flame Retardation of Polymeric Materials
About this book
Flame retardant materials are of vital importance in guaranteeing personal security. Especially the demand for non-toxic, low smoking, polymerized flame retardants increases and new materials enter the market. The authors present the fundamental theory of polymer combustion, compare different flame retardants, describe smoke suppression mechanisms, and explain analyzing techniques for new materials.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Theory of Flame Retardation of Polymeric Materials by Li Jianjun,Ou Yuxiang in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Generally speaking, the combustion process of polymers can be divided into five stages (Figure 1.1) [1] in time, which includes thermal decomposition, ignition, propagation, stable combustion and combustion attenuation. At the same time, these five stages can also be separated in space, such as surface-heating area, condensed phase transformation area (decomposition, crosslinking, carbonization) and vapor-phase combustible combustion area. Figure 1.2 shows the unit model of polymer combustion [2].
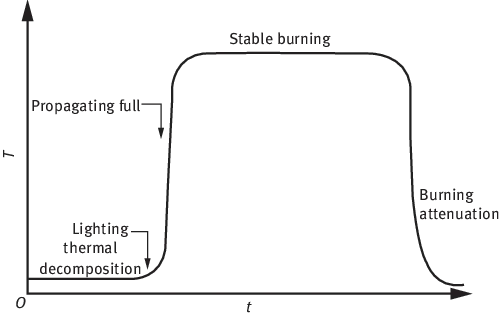
Figure 1.1: Five stages of polymer combustion [1].
Due to their characteristics, there will be some special thermal behaviors of polymer during the heating procedure, such as glass transitions, softening, melting, expansion, foaming, shrinkage, etc [3].
In this chapter, we will not only explain the five stages of polymer combustion but also analyze the smoke, toxic and corrosive products produced during the combustion procedure to provide some theoretical foundations for the discussion of the flame-retardation mechanisms of polymers.
1.1 Thermal decomposition
1.1.1 Overview
When the thermoplastics were heated, evaporation and pyrolysis of the solid phase were considered to be confined to a thin layer, namely condensed-phase/vapor-phase interface. Generally, when the polymer is heated, it will be first thermally degraded into pieces or monomers or fragments with low-molecular weight, and the escape rate of the latter is concerned with the mass transfer process [4, 5].
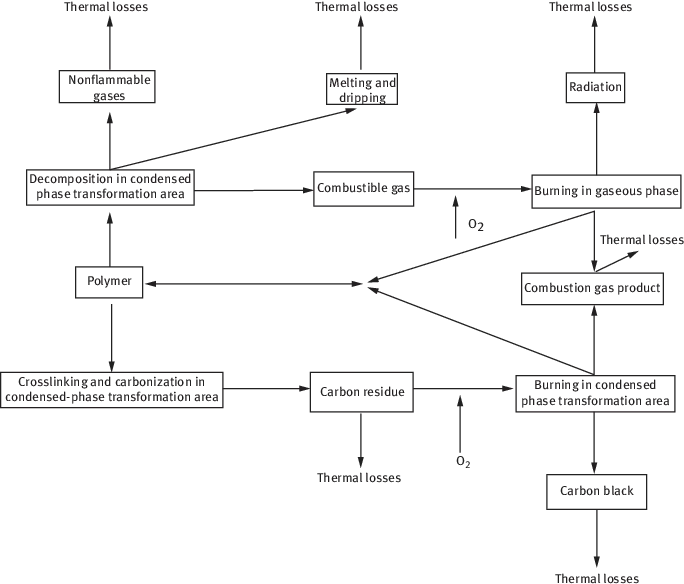
Figure 1.2: Unit model of polymer combustion [2].
The thermal decomposition of polymers is the first step for the cause and development of combustion, and it is very important for estimating the reaction of materials to fire, synthetizing heat-resistant polymer and recycling waste polymer.
When an external heat source is put on the material, the material temperature gradually rises. An external heat source may be produced directly from the flame (by radiation and convective heat transfer), the hot vapor (by conduction and convection heat transfer) and the hot solid material (by heat conduction). When heated, heating rate of material not only depends on external heat flow rate and temperature difference but also is related to specific heat capacity, thermal conductivity and carbonization, evaporation and other changing latent heat of the material.
Under the intense radiation near the hot objects, the surface of polymer will be heated rapidly, and its temperature rises with the square root of thermal radiation time. Taking polyethylene (PE) as an example, when the radiation intensity is 36 kW/M2 (which is equal to the blackbody temperature of 613 °C), its surface temperature rising based on time is shown in Figure 1.3 [6].
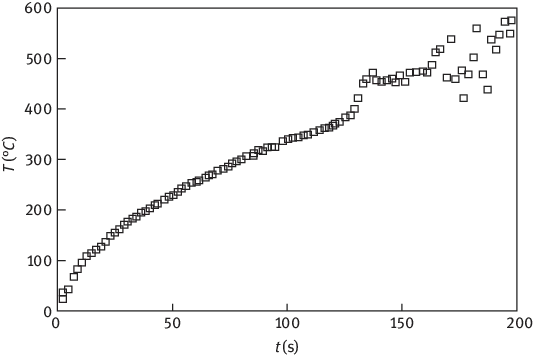
Figure 1.3: Relationship between polyethylene surface temperature and time [6] under a radiation intensity of 36 kW/M2.
If the radiation intensity is low, the highest surface temperature of polymer would not be enough to ignite polymer, as well as would have low thermal diffusivity and thin thermal layer. Thus, polymer is difficult to be ignited (the ignition temperature of polymethyl methacrylate [PMMA] is 250–350 °C, whereas that of PE is 330–370 °C), and only thermal decomposition will occur [7].
When the temperature of polymer rises up to a certain level, it begins to degrade, and the initial temperature of degradation is usually the temperature of bond rupture that has the worst thermal stability. During this period, polymer could still be intact, but the weakest bond ruptures often change the color and luster of polymers. There are two forms of degradation: nonoxide degradation and oxidative degradation. The former occurs without oxygen, whereas the latter should be heated and degraded with oxygen. Proportion of the most volatile decomposition temperature of the bonds and the instability of bonds in polymer are closely related to the degradation of polymer.
When most bonds rupture due to polymer decomposition, it enables a long chain of 104–105 carbon atoms to be decomposed into low molecular product. Then, the molecular weight of polymer gets smaller dramatically, polymer itself also begins to change, and this change can lead to a complete loss on its physical integrity or generate new substance with different properties, most of which are compounds with low molecular weight and many of these compound are inflammable materials, which can also volatilize into the vapor phase, thus reducing the total weight of polymer. For example, when thermal decomposition occurs, polyformaldehyde (POM) or PMMA can be decomposed into monomer formaldehyde or methyl methacrylate, respecti...
Table of contents
- Title Page
- Copyright
- Contents
- Preface
- 1 Polymer combustion
- 2 Flame retardation mechanism of halogenated flame retardants
- 3 The flame-retardation mechanism of organic phosphorus flame retardants
- 4 Flame retardation mechanism of intumescent flame retardant
- 5 Flame retardation mechanism of other flame retardants
- 6 Flame retardation mechanism of polymer, inorganic nanocomposite materials
- 7 The mechanism of smoke suppression
- 8 Technical means on flame retardation mechanism