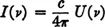![]()
PART I
PHYSICAL FORMULATION OF THE QUANTUM THEORY
MODERN QUANTUM THEORY is unusual in two respects. First, it embodies a set of physical ideas that differ completely with much of our everyday experience, and also with most experiments in physics on a macroscopic scale. Second, the mathematical apparatus needed to apply this theory to even the simplest examples is much less familiar than that required in corresponding problems of classical physics. As a result, there has been a tendency to present the quantum theory as being inseparable from the mathematical problems that arise in its applications. This approach might be likened to introducing Newton’s laws of motion to a student of elementary physics, as problems in the theory of differential equations. In this book, special emphasis is placed on developing the guiding physical principles that are useful not only when it is necessary to apply our ideas to a new problem, but also when we wish to forsee the general properties of the mathematical solutions without carrying out extensive calculations. The development of the special mathematical techniques that are necessary for obtaining quantitative results in complex problems should take place, for the most part, either in a mathematics course or in a special course concerned with the mathematics of quantum theory. It seems impossible, however, to develop quantum concepts extensively without Fourier analysis. It is, therefore, presupposed that the reader is moderately familiar with Fourier analysis.
In the first part of this book, an unusual amount of attention is given to the steps by which the quantum theory may be developed, starting with classical theory and with specific experiments that led to the replacement of classical theory by the quantum theory. The experiments are presented not in historical order, but rather in what may be called a logical order. An historical order would contain many confusing elements that would hide the inherent unity that the quantum theory possesses. In this book, the experimental and theoretical developments are presented in such a way as to emphasize this unity and to show that each new step is either based directly on experiment or else follows logically from the previous steps. In this manner, the quantum theory can be made to seem less like a strange and somewhat arbitrary prescription, justified only by the fact that the results of its abstruse mathematical calculations happen to agree with experiment.
As an integral part of our plan for developing the theory on a basis that is not too abstract for a beginner, a complete account of the relation between quantum theory and the previously existing classical theory is given. Wherever possible the meaning of the quantum theory is illustrated in simple physical terms. Moreover, the final chapter of Part I points out broad regions of everyday experience in which we continually use ways of thinking that are closer to quantum-theoretical than to classical concepts. In this chapter, we also discuss in detail some of the philosophical implications of the quantum theory, and show that these lead to a striking modification in our general view of the world, as compared with that suggested by classical theory.
The reader will notice that most of the problems are interspersed throughout the text. These problems should be read as part of the text, because the results obtained from them are often used directly in the development of ideas. It is usually possible to understand the significance of the results without solving the problems, but the reader is strongly urged to try to solve them. The main advantage of the interspersed problems is that they make the reader think more specifically about the subject previously discussed, thus facilitating his understanding of the subject.
Supplementary References
The following list of supplementary texts will prove very helpful to the reader and will be referred to throughout various parts of this book:
Bohr, N., Atomic Theory and the Description of Nature. London: Cambridge University Press, 1934.
Born, M., Atomic Physics. Glasgow: Blackie & Son, Ltd., 1945.
Born, M., Mechanics of the Atom. London: George Bell & Sons, Ltd., 1927.
Dirac, P. A. M., The Principles of Quantum Mechanics. Oxford : Clarendon Press, 1947.
Heisenberg, W., The Physical Principles of the Quantum Theory. Chicago: University of Chicago Press, 1930.
Kramers, H. A., Die Grundlagen der Quantentheorie. Leipzig: Akademische Verlagsgesellschaft, 1938.
Mott, N. F., An Outline of Wave Mechanics. London: Cambridge University Press, 1934.
Mott, N. F., and I. N. Sneddon, Wave Mechanics and Its Applications. Oxford: Clarendon Press, 1948.
Pauli, W., Die Allgemeinen Prinzipen der Wellenmechanik. Ann Arbor, Mich.: Edwards Bros., Inc., 1946. Reprinted from Handbuch der Physik, 2. Aufl., Band 24. 1. Teil.
Pauling, L., and E. Wilson, Introduction to Quantum Mechanics. New York: McGraw-Hill Book Company, Inc., 1935.
Richtmeyer, F. K., and E. H. Kennard, Introduction to Modern Physics. New York: McGraw-Hill Book Company, Inc., 1933.
Rojansky, V., Introductory Quantum Mechanics. New York: Prentice-Hall, Inc., 1938.
Ruark, A. E., and H. C. Urey, Atoms, Molecules, and Quanta. New York: McGraw-Hill Book Company, Inc., 1930.
Schiff, L., Quantum Mechanics. New York: McGraw-Hill Book Company, Inc., 1949.
![]()
CHAPTER 1
The Origin of the Quantum Theory
The Rayleigh-Jeans Law
1. Blackbody Radiation in Equilibrium. Historically, the quantum theory began with the attempt to account for the equilibrium distribution of electromagnetic radiation in a hollow cavity. We shall, therefore, begin with a brief description of the characteristics of this distribution of radiation. The radiant energy originates in the walls of the cavity, which continually emit waves of every possible frequency and direction, at a rate which increases very rapidly with the temperature. The amount of radiant energy in the cavity does not, however, continue to increase indefinitely with time, because the process of emission is opposed by the process of absorption that takes place at a rate proportional to the intensity of radiation already present in the cavity. In the state of thermodynamic equilibrium, the amount of energy U(v)dv, in the frequency range between v and v + dv, will be determined by the condition that the rate at which the walls emit this frequency shall be balanced by the rate at which they absorb this frequency. It has been demonstrated both experimentally and theoretically,* that after equilibrium has been reached, U(v) depends only on the temperature of the walls, and not on the material of which the walls are made nor on their structure.
To observe this radiation, we make a hole in the wall. If the hole is very small compared with the size of the cavity, it produces a negligible change in the distribution of radiant energy inside the cavity. The intensity of radiation per unit solid angle coming through the hole is then readily shown to be
, where
c is the velocity of light.
†Measurements disclose that, at a particular temperature, the function U(v) follows a curve resembling the solid curve of Fig. 1. At low frequencies the energy is proportional to v2, while at high frequencies it drops off exponentially. As the temperature is raised, the maximum is shifted in the direction of higher frequencies; this accounts for the change in the color of the radiation emitted by a b...