
Electronic Structure and the Properties of Solids
The Physics of the Chemical Bond
- 582 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Electronic Structure and the Properties of Solids
The Physics of the Chemical Bond
About this book
"Should be widely read by practicing physicists, chemists and materials scientists." — Philosophical Magazine
In this comprehensive and innovative text, Professor Harrison (Stanford University) offers a basic understanding of the electronic structure of covalent and ionic solids, simple metals, transition metals, and their compounds. The book illuminates the relationships of the electronic structures of these materials and shows how to calculate dielectric, conducting, and bonding properties for each. Also described are various methods of approximating electronic structure, providing insight and even quantitative results from the comparisons. Dr. Harrison has also included an especially helpful "Solid State Table of the Elements" that provides all the parameters needed to estimate almost any property of any solid, with a hand-held calculator, using the techniques developed in the book.
Designed for graduate or advanced undergraduate students who have completed an undergraduate course in quantum mechanics or atomic and modern physics, the text treats the relation between structure and properties comprehensively for all solids rather than for small classes of solids. This makes it an indispensable reference for all who make use of approximative methods for electronic-structure engineering, semiconductor development and materials science.
The problems at the ends of the chapters are an important aspect of the book. They clearly show that the calculations for systems and properties of genuine and current interest are actually quite elementary. Prefaces. Problems. Tables. Appendixes. Solid State Table of the Elements. Bibliography. Author and Subject Indexes.
"Will doubtless exert a lasting influence on the solid-state physics literature." — Physics Today
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
PART I
ELECTRON STATES
CHAPTER 1
The Quantum-Mechanical Basis
SUMMARY
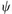
1-A Quantum Mechanics
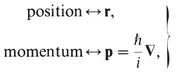
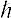
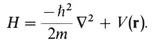
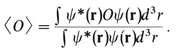
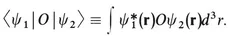
Table of contents
- Title Page
- Copyright Page
- Dedication
- Preface to the Dover Edition - Recent Developments
- Preface to the First Edition
- Table of Contents
- PART I - ELECTRON STATES
- PART II - COVALENT SOLIDS
- PART III - CLOSED-SHELL SYSTEMS
- PART IV - OPEN-SHELL SYSTEMS
- APPENDIX A - The One-Electron Approximation
- APPENDIX B - Nonorthogonality of Basis States
- APPENDIX C - The Overlap Interaction
- APPENDIX D - Quantum-Mechanical Formulation of Pseudopotentials
- APPENDIX E - Orbital Corrections
- Solid State Table of the Elements
- Bibliography and Author Index
- Subject Index