
- 352 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
A leading figure in twentieth-century physics offers impressions and recollections of the field's development. Nobel Laureate Emilio Segrè (1905–89) knew and worked with many of modern physics' preeminent scientists. In this simple but elegant history, he offers compelling views not only of the milestones of scientific discovery but also the personalities involved—their attitudes and politics as well as their trials and triumphs. Highlights include a profile of Albert Einstein, from his unconventional youth to his role as science's elder statesman; the wonder year of 1932, which witnessed the discoveries of the neutron, positron, and deuterium; and the first steps in building particle accelerators.
A student and colleague of Enrico Fermi, Segrè made numerous important contributions to nuclear physics, including participation in the Manhattan Project. Segrè is further renowned for his narrative skills as a historian. This book is a companion to the author's From Falling Bodies to Radio Waves: Classical Physicists and Their Discoveries, also available from Dover Publications.
A student and colleague of Enrico Fermi, Segrè made numerous important contributions to nuclear physics, including participation in the Manhattan Project. Segrè is further renowned for his narrative skills as a historian. This book is a companion to the author's From Falling Bodies to Radio Waves: Classical Physicists and Their Discoveries, also available from Dover Publications.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access From X-rays to Quarks by Emilio Segrè in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Science & Technology Biographies. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
Mathematics and physics are words that often evoke unpleasant memories of concepts that were difficult to understand and appealed to odd individuals. When I went to school, teachers occasionally called science a “dry” subject, and many pupils agreed. The sight of mathematical formulae in print was a sure indication of incomprehensibility or even of black magic. Even now science is so often accused of nefarious doings that one forgets that it has also done some good.
Despite these negative views, scientific research is just as fascinating, dramatic, and full of human interest as artistic creation. However, most of the historical and biographical aspects that are given prominence in literary or artistic disciplines are often omitted in the teaching of science. This is probably because of the cumulative character of science. If there had been no Newton, someone else would have invented calculus and discovered gravitation, but without Shakespeare there would not have been Hamlet. It is thus considered more justifiable to study Shakespeare’s life than Newton’s.
I believe, however, that physics, too, has a rich human component, and it is mainly this element that I want to describe here. I limit myself to physics because it is the field in which I have direct knowledge. I hope this familiarity might enable me to communicate some of the inspiration, the creative effort, and the drama entailed in scientific work.
These historical aspects should interest not only physicists. It has often been said, probably correctly, that the nineteenth and twentieth centuries are an era as brilliant and as unique for science as the Renaissance was for art. He who has had the good fortune to be a contemporary of the Michelangelos or Shakespeares of this age can recall it with an immediacy and pathos that go beyond what can be grasped from the works alone. Although one of the major exponents of this renaissance, Marie Curie, said, “En science nous devons nous intéresser aux choses, non aux personnes” [In science we should concern ourselves with things, not people.], I believe that this judgment is too stern.
In this book I will try to evoke the personalities of some of the major physicists of this century and to point out some of their achievements, attempting to make them understandable to the layman. With some good will, and with some gaps, this is possible. I will avoid becoming so technical as to be intelligible only to the professional. Occasionally readers may skip a few pages if they find them too difficult, without losing the thread of the events.
Some knowledge of physics, however, is necessary. Whereas we can all look at Michelangelo’s David or read Hamlet (and even here there are great differences in appreciation depending on our background), it is not possible to understand the double nature of light quanta or Schrödinger’s equation without some preparation. Mathematical formulae simplify the tale. Mathematics is the natural language of physics, as Galileo pointed out, and although Volta and Faraday wrote great physics without using a formal mathematical language, they thought mathematically, and their ignorance of standard mathematics makes them less, not more, intelligible.
We must never forget that many scientific advances were achieved by the contributions of a multitude of workers, who prepared the terrain and did essential spade work. These people are often unknown or forgotten as individuals, but collectively they are indispensable. Furthermore, scientific events are interrelated and may overlap in time or space. If one tries to follow too closely, this intricate counterpoint can lead to complication and confusion. I have thus chosen to follow the trend of events, sometimes at the expense of strict chronological order.
The Physicists’ World in 1895
It is natural to start our story around 1895 because in two or three years at that time physics took a decisive turn: A few experimental discoveries opened up microscopic consideration of the atomic world. Chemists had known of atoms for at least a hundred years, and through the kinetic theory of gases physicists had also made good use of atomic ideas, but nothing was known about atomic composition and structure.
In the Western world, where the knowledge of atomic structure began to unfold, England, France, and Germany were the three leaders in science. The three big powers were all experiencing different political and social situations. England was at the peak of her splendor under the rule of Victoria Regina and Imperatrix. The queen, who had become Empress of India in 1876, had been on the throne since 1837. The celebration of her jubilee in 1887 turned into a demonstration of the country’s loyalty to her and pride in her empire. Enriched by 2,500,000 square miles of recently acquired territory, Britannia “ruled the waves” in splendid isolation.
France was still smarting from the defeats in the Franco-Prussian war of 1870 and 1871, which had been a tremendous blow to her ego and to the self-image of all French people. The demoralization of the French can be measured by the reaction of Pasteur and other French scientists to the disasters of the war. Distressed, wounded in their deep-rooted patriotism, they associated France’s defeat with her neglect of science over the previous fifty years and recalled with pride the part played by science in the defense of the country during the Revolution and the Napoleonic wars. Pasteur hoped that through science he might be able to hasten France’s recovery.
Germany, rapidly ascending and dominated by the military, had set off on an imperial course. The long struggle between civilian and military authority, which had lasted over sixty years, had been unfortunately resolved with the predominance of the military. Bismarck had been fired in 1890. Kaiser Wilhelm II (1859-1941) was young as rulers go and not experienced. Considering himself very intelligent—which he was not—he believed that he was ruling Germany superbly and bringing her to glorious times. At the start of the First World War he said, “Ich führe euch herrlichen Zeiten entgegen” [I lead you to glorious times“]. So much for his judgment.
In the world of 1895 there were no airplanes, virtually no telephones, and very little electricity. The ocean could be crossed on a steamship, but even then, seventy-five years after transatlantic boats had begun using steam, the steamship was occasionally equipped with supplementary sails. The main form of communication was mail, not only between distant places but also within cities. Paris, for instance, had a quite rapid system of pneumatic mail: a network of pipes in which letters were moved by compressed air. Streets were lit by gas.
In 1895 there were no automobiles; but two years later when Ernest Rutherford visited the exhibition at the Crystal Palace in London, he wrote to his mother, “The chief point of interest to me was the horseless carriage, two of which were practicing on the ground in front.” They traveled at about 12 miles an hour but made “rather a noise and rattle.” However, traffic accidents did happen even in the absence of automobiles, when horses drawing cabs or carts ran out of control; a few years later, in 1906, science was to lose one of its highest exponents to such an accident. There was no smog, but the streets reeked of manure, as inevitable a consequence of the transportation of those times as exhaust fumes are of our gasoline-propelled vehicles. Cities were smaller and more beautiful than at present, but they often had inadequate sanitation.
Physics laboratories were very different in organization and equipment from our present ones. There was usually only one professor, who often had his residence at the laboratory, and who was helped by very few assistants. Now when we rank the facilities of an institution, we do so according to the energy of its accelerator or perhaps the cooling capacity of its cryogenic establishment. But in 1895 accelerators and modern cryogenic plants were far in the future, although the liquefaction of air on a commercial scale had been achieved by that year.
One way of ranking a laboratory was according to the power of the battery it owned. Laboratories in those days needed electricity for experiments, but they could not draw electricity from the mains for the simple reason that mains seldom existed, so they kept batteries in their basements. A battery consisted of a collection of cells; the larger the number of cells, the higher the status of the establishment. Several types of cells had been developed since Volta’s original “electric pile” of 1800. They were all based on the same principle but varied in the composition of their electrodes and in their electrolytic solutions. Many scientific laboratories used Bunsen cells, which could reach a high voltage (up to 1.95 volts) and could deliver heavy currents. But it was quite a task to keep them in working condition. They contained sulfuric acid and nitric acid, which corroded the zinc anode and exuded strong, objectionable fumes.
There is detailed advice for handling batteries of Bunsen cells in a French physics textbook by Adolphe Ganot, published in 1863. (It was the Italian edition of this book that introduced me to physics when I was about eleven years old.) In rereading it recently, I was impressed by the vivid advice, excerpts of which I translate below:
The mixture of water and sulphuric acid must be prepared in advance.... First pour the water into a wooden tub, then add one tenth in volume of common sulphuric acid, so that the solution will indicate 10 to 11 degrees on the Baumé acid scale. If a Baumé scale is not available, the water is sufficiently acidulated when it becomes lukewarm and a drop of it placed on the tongue cannot be held there. Cells must be placed ... on a very dry wooden table.... Then with a funnel pour nitric acid in the porous inner container up to two centimeters from the top.... The truncated cones that fit in the carbon must be carefully cleaned with sandpaper to ensure good connection.... What must be watched above all is the amalgamation of the zinc plates. A plate needs to be amalgamated when a hissing sound is heard in the acidulated water while the pile is not in use ... the acidulated water may also steam and even boil.... To amalgamate the zinc plates ... place them, one after another in an earthenware vase containing some acidulated water and two kilograms of mercury and spread it on the plates with an iron brush....
One of the important instruments of the time was the Ruhmkorff coil (induction coil), which was used to produce high potential differences and long sparks (Figure 1.1). The instrument consisted of two coils wound around a cylindrical iron core and insulated from each other. An electric battery produced a current in the primary coil, and this current was repeatedly interrupted by a breaker. The variation of the primary current induced a current in the secondary coil, creating a potential difference between the terminals of the secondary. Whereas the primary coil was made of thick wire with few turns, the secondary was a thin wire with so many turns that it was miles long. A large Rühmkorff coil of this period, preserved at the Royal Institution of London, has a secondary coil 280 miles long and could make sparks 42 inches long. So the length of sparks, like the power of a battery, could serve as a standard to rank a laboratory.
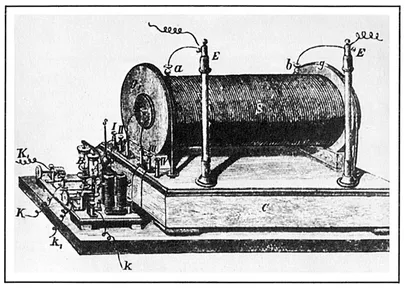
Figure 1.1 A Rühmkorff coil. (From Urbanitzky, Electricity, 1890.) It is a transformer in which the current in the primary coil is suddenly broken. This generates a high voltage in the secondary coil, which gives off a spark in the air. The coil served to supply discharge tubes.
The production of vacuums has dominated physical research for over 100 years, and all the advances in investigation of the atom were coupled with advances in vacuum technology. In the laboratories of 1895 vacuums, created by primitive pumps, were needed for experiments on the discharge of electricity through gases, experiments that resulted in the discovery of x-rays and of the electron not long afterward.
Figure 1.2 shows the pump that was used by Sir William Crookes in his investigations of electrical discharges in vacuum tubes. The tubes to be evacuated were connected to the pump through the drying tube containing phosphoric acid, at the right. The mercury in the container at the left came down the fall tube drop by drop, driving the air out of the apparatus bubble by bubble. The level of the mercury in the gauge tube, compared with the level in a barometer, indicated the degree of vacuum obtained. The mercury reservoir had to be lifted and lowered manually many times, a strenuous task for the technician in charge of evacuating the professor’s tubes and containers. In all such pumps the standard for the perfect vacuum was the barometer. The vacuum attainable with such a pump was about a million times worse than what we would call a decent vacuum today.
To learn in some detail what physicists were doing at the turn of the century, let us take a look in one of the leading journals of the time, the Annalen der Physik. A little earlier, the same journal was entitled Annalen der Physik und Chemie because the sciences of physics and chemistry were still considered jointly, in contrast with our trend toward specialization, which has created a journal for each subbranch of physics. The subjects treated in the Annalen were the liquefaction of gases; the measurement of specific heats; electromagnetic waves, and especially attempts to reproduce with electromagnetic waves all the phenomena of optics; reflection and refraction; diffraction, rotation of the plane of polarization; and so on. Thermodynamics was then about forty years old and not yet entirely consolidated. Gas discharges were studied with the Ruhmkorff coil and with tubes like those shown in Figure 1.6. The kinetic theory of gases was developing vigorously, although not many people were interested in it, and some of the great figures in this field received little recognition. Josiah Willard Gibbs (1839-1903), teaching at Yale University, was ignored by most of the scientific world (except Maxwell and a few others). Ludwig Boltzmann (1844-1908), one of the founders of statistical mechanics, complained in Vienna that nobody in the German-speaking countries was paying attention to his work. Other topics treated in the journals of those times were physical chemistry and ionic dissociation, the beginning of the concept of ions in solution, and the relationship between thermodynamics and chemical equilibrium. No one thought seriously of making models of atoms; not only would this have been beyond feasibility, but the atom had not yet gained full recognition.
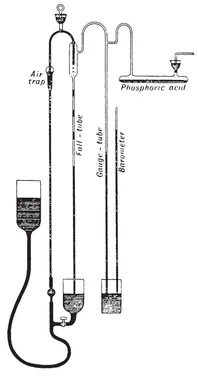
Figure 1.2 A mercury vacuum pump. (From S. P. Thompson, Light Visible and Invisible, 1897.) The pump works by trapping air in the fall tube. The gauge tube compares the vacuum obtained with that in the barometer.
Of course, chemists knew of the atomic “hypothesis,” but belief in the reality of atoms was not shared by all. In retrospect it would seem that since chemists wrote chemical formulae and were acquainted with Avogadro’s law and Faraday’s laws of electrolysis, they should also have believed in atoms. But this was by no means the case. As late as 1905 skepticism was still widespread, with some scientists rejecting outright the corpuscular theory of matter and others recognizing the usefulness of the atomic theory in chemistry but regarding it as remote from reality. These skeptics were neither ...
Table of contents
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Chapter 1 - Introduction
- Chapter 2 - H. Becquerel, the Curies, and the Discovery of Radioactivity
- Chapter 3 - Rutherford in the New World: The Transmutation of Elements
- Chapter 4 - Planck, Unwilling Revolutionary: The Idea of Quantization
- Chapter 5 - Einstein—New Ways of Thinking: Space, Time, Relativity, and Quanta
- Chapter 6 - Sir Ernest and Lord Rutherford of Nelson
- Chapter 7 - Bohr and Atomic Models
- Chapter 8 - A True Quantum Mechanics at Last
- Chapter 9 - The Wonder Year 1932: Neutron, Positron, Deuterium, and Other Discoveries
- Chapter 10 - Enrico Fermi and Nuclear Energy
- Chapter 11 - E. O. Lawrence and Particle Accelerators
- Chapter 12 - Beyond the Nucleus
- Chapter 13 - New Branches from the Old Stump
- Chapter 14 - Conclusions
- Appendix 1 - Stefan’s Law; Wien’s Law
- Appendix 2 - Planck’s Hunt for the Blackbody Radiation Formula
- Appendix 3 - Einstein’s Heuristic Argument for Postulating the Existence of Light Quanta
- Appendix 4 - Brownian Motion
- Appendix 5 - Blackbody Energy Fluctuations According to Einstein
- Appendix 6 - Specific Heat of Solids According to Einstein
- Appendix 7 - A and B of Einstein
- Appendix 8 - J. J. Thomson’s Parabola Method for Finding e/m of Ions
- Appendix 9 - Bohr’s Hydrogen Atom
- Appendix 10 - Quantum Mechanics in a Nutshell
- Bibliography
- Name Index
- Subject Index