eBook - ePub
The VSEPR Model of Molecular Geometry
- 272 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
The VSEPR Model of Molecular Geometry
About this book
Valence Shell Electron Pair Repulsion (VSEPR) theory is a simple technique for predicting the geometry of atomic centers in small molecules and molecular ions. This authoritative reference was written by Istvan Hartiggai and the developer of VSEPR theory, Ronald J. Gillespie. In addition to its value as a text for courses in molecular geometry and chemistry, it constitutes a classic reference for professionals.
Starting with coverage of the broader aspects of VSEPR, this volume narrows its focus to a succinct survey of the methods of structural determination. Additional topics include the applications of the VSEPR model and its theoretical basis. Helpful data on molecular geometries, bond lengths, and bond angles appear in tables and other graphics.
Starting with coverage of the broader aspects of VSEPR, this volume narrows its focus to a succinct survey of the methods of structural determination. Additional topics include the applications of the VSEPR model and its theoretical basis. Helpful data on molecular geometries, bond lengths, and bond angles appear in tables and other graphics.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access The VSEPR Model of Molecular Geometry by Ronald J Gillespie,Istvan Hargittai, Istvan Hargittai in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Inorganic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Topic
Physical SciencesSubtopic
Inorganic Chemistry1
Molecular Geometry
This book is concerned with the geometry of molecules and with the interpretation and prediction of molecular geometry using the valence-shell electron-pair repulsion (VSEPR) model. In this chapter we review some basic ideas and concepts concerning the geometry of molecules. In the following chapter we briefly discuss the more important methods by means of which the geometry of a molecule may be determined. Then in the succeeding chapters we give a detailed discussion of the VSEPR model and use it to discuss the geometry of a wide variety of molecules. In the last chapter we consider the theoretical basis of the model and compare it with other models for rationalizing and predicting molecular geometry.
ATOMS, MOLECULES, AND BONDS
A molecule consists of a discrete group of two or more atoms held together in a definite geometrical arrangement. Whenever two or more atoms are held together sufficiently strongly to form a molecule, we say that there are chemical bonds between each atom and its close neighbors. The geometry of a molecule has a profound influence on its properties, and so ever since van’t Hoff and le Bel proposed in 1874 that the bonds formed by a carbon atom have a tetrahedral arrangement the geometry of molecules has been of great interest. Chemists have for a long time represented a bond by a single line. We draw structures for molecules that indicate how it is believed that the atoms are connected together by bonds. We will use the term structure in this sense to indicate simply the connectivity of the atoms, while by geometry we mean the actual three-dimensional arrangement of the atoms.
Each atom in a molecule consists of a positively charged nucleus surrounded by a number of negatively charged electrons. Thus there are two important and closely related questions that we can ask about a molecule:
- What are the relative positions of the nuclei in space? In other words, what is the geometry of the molecule? The geometry of a molecule is usually described in terms of the distances between the atomic nuclei that are bonded together (that is, the bond lengths), the angles between the bonds formed by each atom (that is, the bond angles), and the angles between the bonds on adjacent atoms (that is, the torsional angles).
- How are the electrons arranged? Because electrons are in constant motion and because their paths cannot be precisely defined, the arrangement of the electrons in a molecule is described in terms of an electron density distribution. The electrons in an atom are arranged in successive shells surrounding the nucleus. The nucleus and the inner shells of electrons usually remain unchanged in molecule formation, and it is only the outer shell, known as the valence shell, that is modified. Thus the atomic nucleus and the inner electron shells are considered to constitute the core of the atom so that a molecule is thought of as consisting of two or more positively charged atomic cores held together by the electrostatic attraction of a negatively charged electron density distribution derived from the valence-shell electrons of the constituent atoms. Thus the relationship between the arrangement of the electrons, that is, the electron density distribution, and the bonds that are imagined to hold atoms together in a molecule is of fundamental importance to the understanding of molecular geometry.
The arrangement of the nuclei in a molecule may be determined by several different experimental methods, the most important of which is X-ray diffraction by crystalline solids, as will be described in Chapter 2. The geometry of a molecule may also be found, at least in principle, by determining by quantum mechanical calculations the arrangement of the nuclei that has the minimum energy, as will be discussed in Chapters 2 and 7.
The electron density distribution of a molecule can be determined by the same quantum mechanical calculations as are used to find the energy and geometry of a molecule. But in practice it is only possible to carry out these calculations to a reasonable accuracy for small molecules consisting of light atoms.
The electron density distribution can also, at least in principle, be determined by X-ray diffraction studies on crystalline solids. X-rays are diffracted by the periodically varying electron density distribution in a crystal; but most of the electron density is concentrated in the atomic cores, and so, although it is relatively simple to determine the positions of the atomic cores and therefore of the nuclei, it is often very difficult to detect the small changes in the electron density distribution that occur on molecule formation.
LEWIS STRUCTURES
The arrangement of the electrons in an atom is usually described in terms of its electron configuration as deduced from atomic spectroscopy, ionization energies, and the periodic table, and also from quantum mechanics (Chapter 7). The electron configurations of the elements are given in Table 1.1.
TABLE 1.1 ELECTRON CONFIGURATIONS OF THE ELEMENTS

One of the earliest models of the arrangement of the electrons in molecules is that published by G. N. Lewis in 1916, although it had been used by him in teaching for a number of years before. Chemists have found this model so convenient that it is still today the most widely used simple model. Lewis represented the core of an atom by its symbol and the valence-shell electrons by the appropriate number of dots. Lewis dot diagrams for the main-group elements of the first six periods are given in Figure 1.1.
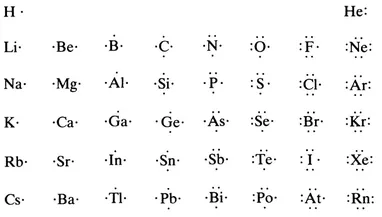
Figure 1.1 Lewis symbols for the main-group elements.
Lewis proposed that in compound formation atoms achieve noble gas electron configurations either by electron loss or gain or by the sharing of one or more electron pairs. Because each of the noble gases, except helium, has eight electrons in its outer or valence shell, Lewis’s proposal is often called the octet rule. Electrons are readily removed from the valence shell of an atom of a metal to give an ion with a noble gas electron configuration; for example,
Na(2s22s22p63s1) → Na+(1s22s22p6) + e-
Nonmetals tend to g...
Table of contents
- Title Page
- Copyright Page
- Table of Contents
- Introduction to the Dover Edition
- Select Bibliography
- Preface
- 1 - Molecular Geometry
- 2 - The Determination of Molecular Geometry
- 3 - The Valence-shell Electron-pair Repulsion Model
- 4 - The Second-period Elements
- 5 - The Main-Group Elements of the Third and Subsequent Periods
- 6 - The Transition Metals
- 7 - The Quantum Mechanical Basis of the VSEPR Model
- Subject Index
- Formula Index
- A CATALOG OF SELECTED DOVER BOOKS IN SCIENCE AND MATHEMATICS