
eBook - ePub
Fundamentals of Chemical Reaction Engineering
- 384 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fundamentals of Chemical Reaction Engineering
About this book
Appropriate for a one-semester undergraduate or first-year graduate course, this text introduces the quantitative treatment of chemical reaction engineering. It covers both homogeneous and heterogeneous reacting systems and examines chemical reaction engineering as well as chemical reactor engineering. The authors take a chemical approach, helping students develop an intuitive feeling for concepts, rather than an engineering approach, which tends to overlook the inner workings of systems and objects.
Each chapter contains numerous worked-out problems and real-world vignettes involving commercial applications. Topics include the basics of reaction kinetics and rate constants of elementary reactions, reactors for measuring reaction rates and the steady-state approximation, and heterogeneous catalysis. Additional subjects include the effects of transport limitations on rates of solid-catalyzed reactions, microkinetic analysis of catalytic reactions, nonideal flow in reactors, nonisothermal reactors, and reactors accomplishing heterogeneous reactions. Excellent illustrations complement the text, which concludes with three helpful appendices.
Each chapter contains numerous worked-out problems and real-world vignettes involving commercial applications. Topics include the basics of reaction kinetics and rate constants of elementary reactions, reactors for measuring reaction rates and the steady-state approximation, and heterogeneous catalysis. Additional subjects include the effects of transport limitations on rates of solid-catalyzed reactions, microkinetic analysis of catalytic reactions, nonideal flow in reactors, nonisothermal reactors, and reactors accomplishing heterogeneous reactions. Excellent illustrations complement the text, which concludes with three helpful appendices.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Fundamentals of Chemical Reaction Engineering by Mark E. Davis,Robert J. Davis, Robert J. Davis in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
The Basics of Reaction
Kinetics for Chemical
Reaction Engineering
1.1 | The Scope of Chemical Reaction Engineering
The subject of chemical reaction engineering initiated and evolved primarily to accomplish the task of describing how to choose, size, and determine the optimal operating conditions for a reactor whose purpose is to produce a given set of chemicals in a petrochemical application. However, the principles developed for chemical reactors can be applied to most if not all chemically reacting systems (e.g., atmospheric chemistry, metabolic processes in living organisms, etc.). In this text, the principles of chemical reaction engineering are presented in such rigor to make possible a comprehensive understanding of the subject. Mastery of these concepts will allow for generalizations to reacting systems independent of their origin and will furnish strategies for attacking such problems.
The two questions that must be answered for a chemically reacting system are: (1) what changes are expected to occur and (2) how fast will they occur? The initial task in approaching the description of a chemically reacting system is to understand the answer to the first question by elucidating the thermodynamics of the process. For example, dinitrogen (N2) and dihydrogen (H2) are reacted over an iron catalyst to produce ammonia (NH3):
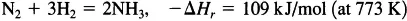
where ΔHr is the enthalpy of the reaction (normally referred to as the heat of reaction). This reaction proceeds in an industrial ammonia synthesis reactor such that at the reactor exit approximately 50 percent of the dinitrogen is converted to ammonia. At first glance, one might expect to make dramatic improvements on the production of ammonia if, for example, a new catalyst (a substance that increases the rate of reaction without being consumed) could be developed. However, a quick inspection of the thermodynamics of this process reveals that significant enhancements in the production of ammonia are not possible unless the temperature and pressure of the reaction are altered. Thus, the constraints placed on a reacting system by thermodynamics should always be identified first.
VIGNETTE 1.1.1
The initial success of a large-scale catalytic technology began in 1913 when the first industrial chemical reactor to synthesize ammonia from dinitrogen and dihydrogen began operation in Germany. Most of the ammonia manufactured today is used to produce nitrogen-rich fertilizers that have an enormous impact on meeting worldwide food demands. According to figures for U.S. agriculture, the 800,000 tons of dinitrogen converted to ammonia in the first Haber reactor (ammon...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Dedication
- Contents
- Preface
- Nomenclature
- Chapter 1: The Basics of Reaction Kinetics for Chemical Reaction Engineering
- Chapter 2: Rate Constants of Elementary Reactions
- Chapter 3: Reactors for Measuring Reaction Rates
- Chapter 4: The Steady-State Approximation: Catalysis
- Chapter 5: Heterogeneous Catalysis
- Chapter 6: Effects of Transport Limitations on Rates of Solid-Catalyzed Reactions
- Chapter 7: Microkinetic Analysis of Catalytic Reactions
- Chapter 8: Nonideal Flow in Reactors
- Chapter 9: Nonisothermal Reactors
- Chapter 10: Reactors Accomplishing Heterogeneous Reactions
- Appendix A: Review of Chemical Equilibria
- Appendix B: Regression Analysis
- Appendix C: Transport in Porous Media
- Back Cover