
Organic Chemistry Workbook
Theory, Reactivity and Mechanisms in Modern Synthesis
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Organic Chemistry Workbook
Theory, Reactivity and Mechanisms in Modern Synthesis
About this book
Provides references and answers to every question presented in the primary Organic Chemistry textbook
Successfully achieving chemical reactions in organic chemistry requires a solid background in physical chemistry. Knowledge of chemical equilibria, thermodynamics, reaction rates, reaction mechanisms, and molecular orbital theory is essential for students, chemists, and chemical engineers. The Organic Chemistry presents the tools and models required to understand organic synthesis and enables the efficient planning of chemical reactions.
This volume, Organic Chemistry: Theory, Reactivity, and Mechanisms in Modern Synthesis Workbook, complements the primary textbook—supplying the complete, calculated solutions to more than 800 questions on topics such as thermochemistry, pericyclic reactions, organic photochemistry, catalytic reactions, and more. This companion workbook is indispensable for those seeking clear, in-depth instruction on this challenging subject.
Written by prominent experts in the field of organic chemistry, this book:
- Works side-by-side with the primary Organic Chemistry textbook
- Includes chapter introductions and re-stated questions to enhance efficiency
- Features clear illustrations, tables, and figures
- Strengthens reader?s comprehension of key areas of knowledge
Organic Chemistry: Theory, Reactivity, and Mechanisms in Modern Synthesis Workbook is a must-have resource for anyone using the primary textbook.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Equilibria and Thermochemistry
Answers to Problems

- Problem 1.2Define the symmetry numbers, σ, of ethane, propane, cyclopropane, cyclobutane, cyclohexanone, ferrocene, bicyclo[2.2.1]hepta‐2,5‐diene (norbornadiene), 1,4‐difluorobenzene, meso‐tartaric acid, and (R,R)‐tartaric acid (see Figure 1.24 for the structure of the two latter compounds).Answer
- Ethane is a flexible molecule that adopts a preferred staggered conformation (c. 3 kcal mol−1 more stable than the eclipsed conformation (Section 2.5.1), transition state of the rotation about its σ(CC) bond). Ethane has a C3‐ and three C2‐axes that are interconverted by rotation about the C3‐axis. Thus, the symmetry number σ = 3 × 2 = 6.
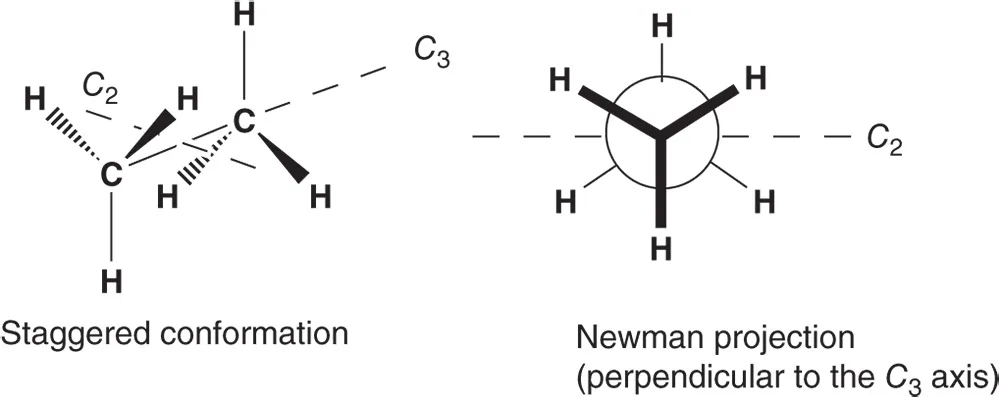 Propane is a flexible molecule for which the doubly staggered conformation is the most stable one (C2v symmetry: one mirror plane of symmetry containing a C2‐axis of rotation) has one C2‐axis of rotation. Thus, the symmetry number σ = 2.
Propane is a flexible molecule for which the doubly staggered conformation is the most stable one (C2v symmetry: one mirror plane of symmetry containing a C2‐axis of rotation) has one C2‐axis of rotation. Thus, the symmetry number σ = 2.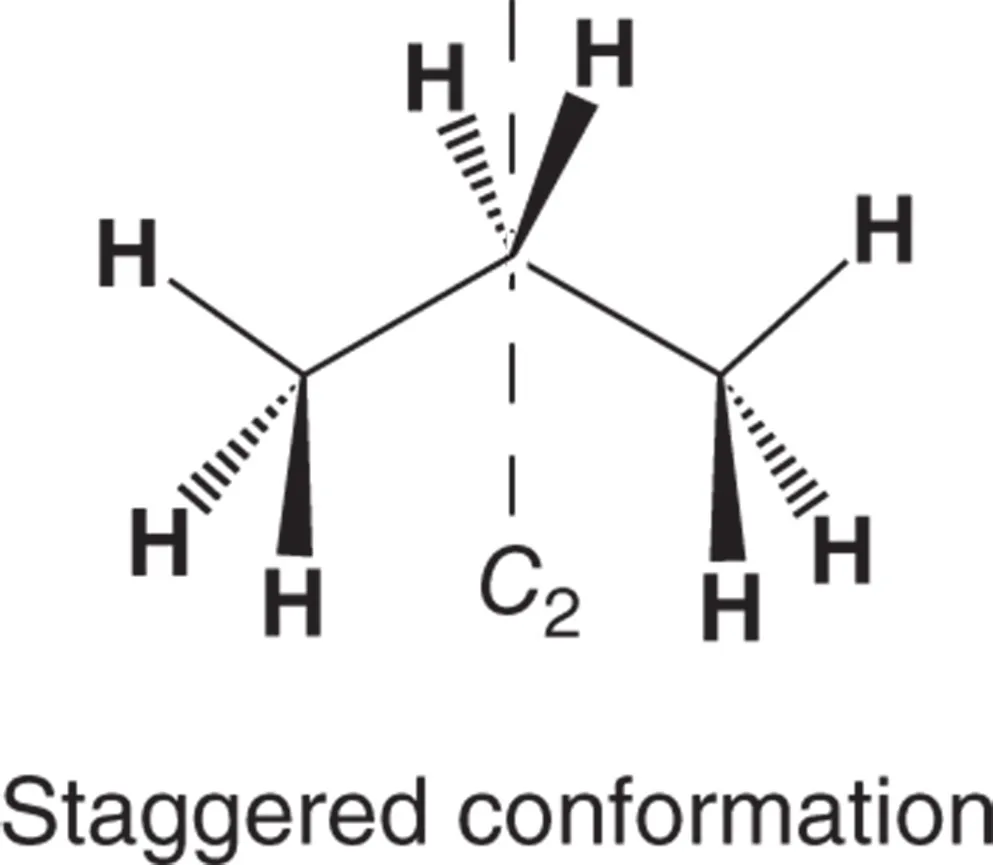 Cyclopropane is a rigid molecule (D3h symmetry) with a C3‐ and three C2‐axes of rotation. Rotation about the C3 axis interconverts the C2‐axis. Thus, the symmetry numb...
Cyclopropane is a rigid molecule (D3h symmetry) with a C3‐ and three C2‐axes of rotation. Rotation about the C3 axis interconverts the C2‐axis. Thus, the symmetry numb...
Table of contents
- Cover
- Table of Contents
- 1 Equilibria and Thermochemistry
- 2 Additivity Rules for Thermodynamical Parameters and Deviations
- 3 The Rates of Chemical Reactions*
- 4 Molecular Orbital Theories
- 5 Pericyclic Reactions*
- 6 Organic Photochemistry
- 7 Catalytic Reactions
- 8 Transition‐Metal‐Catalyzed CC Bond Forming Reactions
- End User License Agreement