
From Deep Sea to Laboratory 3
From Tait's Work on the Compressibility of Seawater to Equations-of-State for Liquids
- English
- ePUB (mobile friendly)
- Available on iOS & Android
From Deep Sea to Laboratory 3
From Tait's Work on the Compressibility of Seawater to Equations-of-State for Liquids
About this book
The scientific expedition of H.M.S. Challenger in the 1870s marks the starting point of physical oceanography. This ship traveled the seas of the globe pursuing a dual objective: to conduct an in-depth study of animal life and to observe the physical properties of ocean waters. Volume 3 focuses on measurements and modeling of liquid compressibility. Based on the work initiated by the physicist Peter Tait, a detailed presentation of liquid equations-of-state is proposed. The physical interpretation of the parameters of these equations is discussed, leading to a description of the "structure" of liquid media. From Deep Sea to Laboratory is available in three volumes for curious readers drawn to travel, history and science. Students, researchers and teachers of physics, fluid mechanics and oceanography will find material to deepen their knowledge.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
The Compressibility of Liquids and Tait’s Equation-of-State
1.1. Introduction
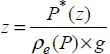
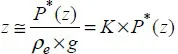
1.2. Concepts of compressibility
Table of contents
- Cover
- Table of Contents
- Foreword
- Preface
- Notations
- 1 The Compressibility of Liquids and Tait’s Equation-of-State
- 2 Interpretations of the Parameters of Tait’s Equation
- 3 Tait-Tammann-Gibson Equations-of-State
- 4 The Modified Tait Equation
- Conclusion Overview and Contributions of Tait’s Work
- Appendices
- References
- Index
- Summary of Volume 1
- Summary of Volume 2
- End User License Agreement