
- 272 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
AP® Chemistry Crash Course Book + Online
About this book
REA's Crash Course for the AP® Chemistry Exam - Gets You a Higher Advanced Placement® Score in Less Time
Crash Course is perfect for the time-crunched student, the last-minute studier, or anyone who wants a refresher on the subject.
Are you crunched for time? Have you started studying for your Advanced Placement® Chemistry exam yet? How will you memorize everything you need to know before the test? Do you wish there was a fast and easy way to study for the exam AND boost your score?
If this sounds like you, don't panic. REA's Crash Course for AP® Chemistry is just what you need. Our Crash Course gives you:
Targeted, Focused Review - Study Only What You Need to Know
Fully revised for the 2014 AP® Chemistry exam, this Crash Course is based on an in-depth analysis of the revised AP® Chemistry course description outline and sample AP® test questions. It covers only the information tested on the new exam, so you can make the most of your valuable study time. Our targeted review focuses on the Big Ideas that will be covered on the exam. Explanations of the AP® Chemistry Labs are also included.
Expert Test-taking Strategies
This Crash Course presents detailed, question-level strategies for answering both the multiple-choice and essay questions. By following this advice, you can boost your score in every section of the test.
Take REA's Online Practice Exam
After studying the material in the Crash Course, go to the online REA Study Center and test what you've learned. Our practice exam features timed testing, detailed explanations of answers, and automatic scoring analysis. The exam is balanced to include every topic and type of question found on the actual AP® exam, so you know you're studying the smart way.
Whether you're cramming for the test at the last minute, looking for extra review, or want to study on your own in preparation for the exams - this is the study guide every AP® Chemistry student must have.
When it's crucial crunch time and your Advanced Placement® exam is just around the corner, you need REA's Crash Course for AP® Chemistry!
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
PART I
INTRODUCTION
Chapter 1
Keys for Success on the AP Chemistry Exam
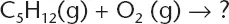
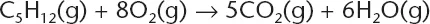
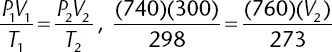
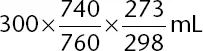
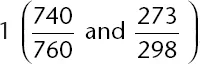
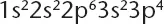
Table of contents
- Cover
- Title Page
- Copyright Page
- Contents
- About This Book
- About Our Author
- Acknowledgments
- PART I Introduction
- PART II Atoms and Elements
- PART III Bonding
- PART IV Chemical Reactions
- PART V Rates of Reaction
- PART VI Chemical Thermodynamics
- PART VII Equilibrium
- PART VIII Overarching Themes
- Periodic Table of the Elements
- AP Chemistry Equations and Constants