
- 240 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Chemistry Super Review - 2nd Ed.
About this book
REA's Chemistry Super Review
Get all you need to know with Super Reviews!
2nd Edition
REA's Chemistry Super Review contains an in-depth review that explains everything high school and college students need to know about the subject. Written in an easy-to-read format, this study guide is an excellent refresher and helps students grasp the important elements quickly and effectively.Our Chemistry Super Review can be used as a companion to high school and college textbooks, or as a handy resource for anyone who wants to improve their chemistry skills and needs a fast review of the subject.Presented in a straightforward style, our review covers the material taught in a beginning-level chemistry course, including: atomic structure, bonding, chemical reactions, liquids, solids, gases, properties of solutions, chemical thermodynamics, and more.
The book contains questions and answers to help reinforce what students learned from the review. Quizzes on each topic help students increase their knowledge and understanding and target areas where they need extra review and practice.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
CHAPTER 1
Introduction
1.1 Matter and Its Properties
1.1.1 Definition of Matter
1.1.2 States of Matter
1.1.3 Composition of Matter
1.1.4 Properties of Matter
1.2 Conservation of Matter
1.2.1 Law of Conservation of Matter
1.3 Laws of Definite and Multiple Proportions
1.3.1 Law of Definite Proportions

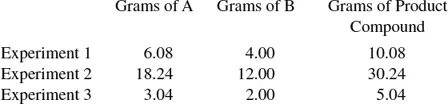

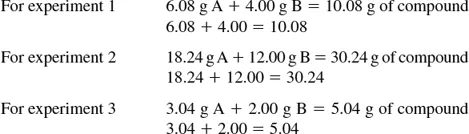
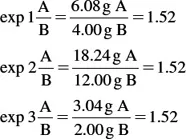
Table of contents
- Cover Page
- Title Page
- Copyright Page
- REA’s Chemistry Super Review®
- Available Super Review® Titles
- About Research & Education Association
- Contents
- Chapter 1 Introduction
- Chapter 2 Stoichiometry, Chemical Arithmetic
- Chapter 3 Atomic Structure and the Periodic Table
- Chapter 4 Bonding
- Chapter 5 Chemical Formulas
- Chapter 6 Types and Rates of Chemical Reactions
- Chapter 7 Gases
- Chapter 8 Liquids, Solids, and Phase Changes
- Chapter 9 Properties of Solutions
- Chapter 10 Acids and Bases
- Chapter 11 Acid-Base Equilibria in Aqueous Solutions
- Chapter 12 Chemical Equilibrium
- Chapter 13 Chemical Thermodynamics
- Chapter 14 Oxidation and Reduction
- Back Cover