
eBook - ePub
Organosilicon Chemistry
Novel Approaches and Reactions
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Organosilicon Chemistry
Novel Approaches and Reactions
About this book
Provides a unique summary of important catalytic reactions in the presence of silicon
A must-have for all synthetic chemists, this book summarizes all of the important developments in the application of organosilicon compounds in organic synthesis and catalysis. Edited by two world leaders in the field, it describes different approaches and covers a broad range of reactions, e.g. catalytic generation of silicon nucleophiles, Si-H Bond activation, C-H bond silylation, silicon-based cross-coupling reactions, and hydrosilylation in the presence of earth-abundant metals.
In addition to the topics covered above, Organosilicon Chemistry: Novel Approaches and Reactions features chapters that look at Lewis base activation of silicon Lewis acids, silylenes as ligands in catalysis, and chiral silicon molecules.
-The first book about this topic in decades, covering a broad range of reactions
-Covers new approaches and novel catalyst systems that have been developed in recent years
-Written by well-known, international experts in the areas of organometallic silicon chemistry and organosilicon cross-coupling reactions
Organosilicon Chemistry: Novel Approaches and Reactions is an indispensable source of information for synthetic chemists in academia and industry, working in the field of organic synthesis, catalysis, and main-group chemistry.
A must-have for all synthetic chemists, this book summarizes all of the important developments in the application of organosilicon compounds in organic synthesis and catalysis. Edited by two world leaders in the field, it describes different approaches and covers a broad range of reactions, e.g. catalytic generation of silicon nucleophiles, Si-H Bond activation, C-H bond silylation, silicon-based cross-coupling reactions, and hydrosilylation in the presence of earth-abundant metals.
In addition to the topics covered above, Organosilicon Chemistry: Novel Approaches and Reactions features chapters that look at Lewis base activation of silicon Lewis acids, silylenes as ligands in catalysis, and chiral silicon molecules.
-The first book about this topic in decades, covering a broad range of reactions
-Covers new approaches and novel catalyst systems that have been developed in recent years
-Written by well-known, international experts in the areas of organometallic silicon chemistry and organosilicon cross-coupling reactions
Organosilicon Chemistry: Novel Approaches and Reactions is an indispensable source of information for synthetic chemists in academia and industry, working in the field of organic synthesis, catalysis, and main-group chemistry.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Organosilicon Chemistry by Tamejiro Hiyama, Martin Oestreich, Tamejiro Hiyama,Martin Oestreich in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Catalytic Generation of Silicon Nucleophiles
Koji Kubota and Hajime Ito
Institute for Chemical Reaction Design and Discovery (WPI‐ICReDD), Hokkaido University, Sapporo, Hokkaido, 060‐8628, Japan
Faculty of Engineering, Division of Applied Chemistry and Frontier Chemistry Center, Hokkaido University, Kita 13 Nishi 8, Kita‐ku, Sapporo, Hokkaido, 060‐8628, Japan
1.1 Introduction
Silicon nucleophiles represent a class of important organometallic species for silicon–carbon, silicon–silicon, and silicon–boron bond formation reactions in synthetic chemistry [1]. Conventionally, the generation of silicon nucleophiles is accomplished by reactions of chlorosilanes with alkali metal (K, Na, Li), reactions of hydrosilanes with alkali metal hydride, cleavage of the silicon–silicon bond in disilanes or the silicon–boron bond in silylboron reagents by organometallic carbon nucleophiles, and transmetallation from other silicon‐metal compounds [2]. However, these stoichiometric methods have significant limitations such as low functional‐group compatibility due to the high reactivity of hard silyl anions with an alkali metal countercation. In this context, silicon‐based organocuprates are widely used as soft silyl anion equivalents for silicon–carbon bond formation reactions, even though this method requires stoichiometric organometallic compounds and copper salt [3]. Recently, catalytic nucleophilic silylation reactions have attracted considerable attention because of their mild reaction conditions and unique selectivity and reactivity. This chapter mainly focuses on two types of activation modes for catalytic generation of silicon nucleophiles (Figure 1.1). First, transmetalation between silicon compounds containing a Si─X bond (X = Si, B, and Zn) and metal catalysts generates nucleophilic silyl metal intermediates (Figure 1.1a). Second, a catalytic amount of Lewis bases (Nu) activates the silicon–boron bond of silylboron reagents to form nucleophilic silyl species (Figure 1.1b). This chapter provides the recent advancements in the catalytic generation of silicon nucleophiles through these activation pathways and their applications in organic synthesis.

Figure 1.1 Representative pathways for catalytic generation of silicon nucleophiles. (a) Metal‐catalyzed method. (b) Lewis base‐catalyzed method.
1.2 Silicon Nucleophiles with Copper Catalysts
1.2.1 Copper‐Catalyzed Nucleophilic Silylation with Disilanes
1.2.1.1 Silylation of α,β‐Unsaturated Carbonyl Compounds
In 1998, the first example of copper‐catalyzed nucleophilic 1,4‐silylation of α,β‐unsaturated carbonyl compounds with disilanes was reported by Ito et al. (Scheme 1.1) [4]. The reaction of cyclohexanone with a disilane in the presence of a copper salt and Bu3P as a ligand proceeded to give the corresponding 1,4‐silyl addition product in high yield. The silylation presumably goes through the σ‐bond metathesis between a copper salt and a disilane to form the silylcopper intermediate, followed by its 1,4‐addition to cyclohexanone. The copper catalyst is regenerated by the reaction between the resultant copper enolate and silyl triflate, which is formed at the first stage of this cycle. This mild protocol can be applied to a variety of substrates such as α,β‐unsaturated cyclic and linear ketones and aldehydes to form the β‐silyl carbonyl compounds in high yields.

Scheme 1.1 Copper‐catalyzed silylation of α,β‐unsaturated carbonyl compounds with a disilane.
1.2.1.2 Silylation of Alkylidene Malonates
Scheidt and coworkers reported the copper‐catalyzed nucleophilic silylation of alkylidene malonates with disilanes in 2004 (Scheme 1.2) [5]. They found pyridine to be an effective ligand rather than phosphines for this reaction.
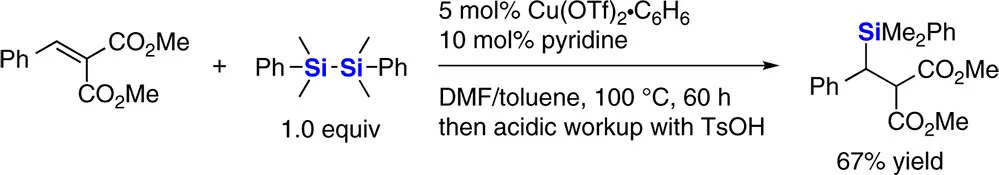
Scheme 1.2 Copper‐catalyzed silylation of alkylidene malonates with a disilane.
1.2.1.3 Silylation of Allylic Carbamates...
Table of contents
- Cover
- Table of Contents
- Foreword
- Preface
- 1 Catalytic Generation of Silicon Nucleophiles
- 2 Si─H Bond Activation by Main‐Group Lewis Acids
- 3 Si─H Bond Activation by Transition‐Metal Lewis Acids
- 4 Metal–Ligand Cooperative Si─H Bond Activation
- 5 Cationic Silicon‐Based Lewis Acids in Catalysis
- 6 Transition‐Metal‐Catalyzed C─H Bond Silylation
- 7 Transition‐Metal‐Free Catalytic C─H Bond Silylation
- 8 Silyl‐Heck, Silyl‐Negishi, and Related Reactions
- 9 Transition‐Metal‐Catalyzed Cross‐coupling of Organosilicon Compounds
- 10 Lewis Base Activation of Silicon Lewis Acids
- 11 Hydrosilylation Catalyzed by Base Metals
- 12 Silylenes as Ligands in Catalysis
- 13 Enantioselective Synthesis of Silyl Ethers Through Catalytic Si─O Bond Formation
- 14 Chiral Silicon Molecules
- Index
- End User License Agreement