
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Offers a physical organic chemistry and mechanistic perspective of the chemistry of thermal processes in the gas phase
The book looks at all aspects of the chemical processing technique called gas-phase pyrolysis, including its methodology and reactors, synthesis, reaction mechanisms, structure, kinetics, and applications. It discusses combinations of pyrolytic reactors with physiochemical techniques, routes for and reactions for the synthesis of organic compounds, and the control of reaction rates.
Gas-Phase Pyrolytic Reactions: Synthesis, Mechanisms, and Kinetics starts with in-depth chapter coverage of static pyrolysis, dynamic flow pyrolysis, and analytical pyrolysis. It then examines synthesis and applications, including flash vacuum pyrolysis in organic synthesis, elimination of HX, elimination of CO and CO 2, pyrolysis of Meldrum's acid derivatives, and elimination of N 2. A chapter on reaction mechanism comes next and includes coverage of retero-ene reaction and reactive intermediates. Following that are sections covering: structure/reactivity correlation, functional group & structural frame interconversions; gas-phase pyrolysis of hydrazones and phosphorus Ylides; and more.
- Deals with a growing area of chemistry and engineering interest that fits under the practices of green and sustainable chemistry
- Addresses several important aspects: methodology and reactors, synthesis, reaction mechanisms, structure, kinetics, and applications
- Reviews general methods of pyrolysis techniques
- Sets out the fundamentals and advantages of gas-phase pyrolysis in a way that illustrates its wide potential applications
Gas-Phase Pyrolytic Reactions: Synthesis, Mechanisms, and Kinetics will appeal to organic chemists, physical organic chemists, chemical engineers and anyone interested in green/sustainable chemistry, chemical synthesis, or process chemistry.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Methodologies and Reactors
Chapter Menu
- Static Pyrolysis
- Sealed-Tube Reactor
- Static Apparatus
- Dynamic Flow Pyrolysis
- Flash Vacuum Pyrolysis
- Synthetic Applications of FVP
- Gas-Flow Pyrolysis vs. STP
- Limitations of FVP
- Spray Pyrolysis
- Falling-Solid Pyrolysis
- Analytical Pyrolysis
- Pyrolysis Gas Chromatography (Py-GC)
- Pyrolysis Mass Spectrometry
- FVP with Spectroscopy
- Catalytic Gas-Phase Pyrolysis
- References
1.1 Static Pyrolysis
1.1.1 Sealed‐Tube Reactor
1.1.1.1 Pyrolyzer
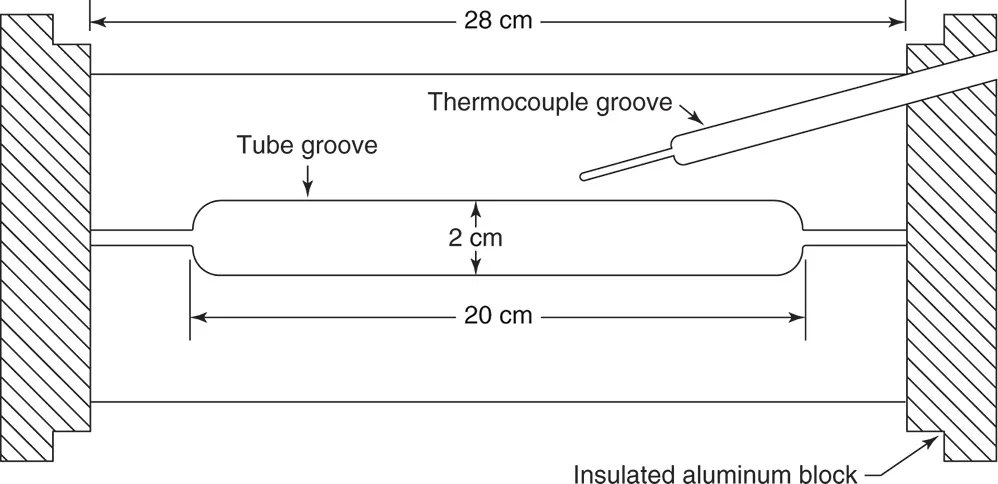
1.1.1.2 Reaction Tube

1.1.1.3 Kinetic Studies
Table of contents
- Cover
- Table of Contents
- Preface
- List of Abbreviations
- About the Author
- 1 Methodologies and Reactors
- 2 Synthesis and Applications
- 3 Reaction Mechanism
- 4 Structure/Reactivity Correlation
- 5 Functional Group and Structural Frame Interconversions
- 6 Gas‐Phase Pyrolysis of Hydrazones
- 7 Gas‐Phase Pyrolysis of Phosphorus Ylides
- Index
- End User License Agreement