
Synthetic Polymer Chemistry
Innovations and Outlook
- 352 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Synthetic Polymer Chemistry
Innovations and Outlook
About this book
Polymeric materials form the basis of daily life. Despite the great contribution of traditional methodologies such as anionic and radical polymerizations in preparing various functional polymers, the increasing demand for polymers with new structures and functions has inspired the development of new synthetic techniques. Many new polymerizations including click polymerization, controlled/living radical polymerization, multicomponent polymerization have been well developed. Focusing on breakthroughs and recent progress, Synthetic Polymer Chemistry provides efficient tools for the synthesis of linear and topological polymers. Chapters cover topics including fabrication of supramolecular polymers, organocatalytic synthesis and olefin co(polymerization). This title will be a valuable reference for those working in polymer chemistry, as well as students and researchers interested in opto-electronic, biological and materials sciences.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
*E-mail: [email protected], [email protected]
Sulfur(vi) Fluoride Exchange (SuFEx) click chemistry was reported by Sharpless et al. in 2014. The applications of SuFEx click chemistry have been demonstrated in various disciplines since then, including the development of synthetic methods and the preparation of polymers. Fluorosulfates have also been found to be unique warheads for covalent capture of protein side chains in a highly selective, context-dependent manner. A review of the story of discovering SuFEx will be included in this chapter. We will also focus on the details demonstrating the whole process to synthesize new polysulfonate and polysulfate polymers, especially the evolution of the catalysts involved. Aside from leading to the discovery of new polymers, SuFEx click chemistry as a powerful tool in polymer modification will also be discussed herein.
1.1 Introduction
1.1.1 Reactivity isn't Everything, It is the Only Thing
It is the only thing.”
It is the only thing.”
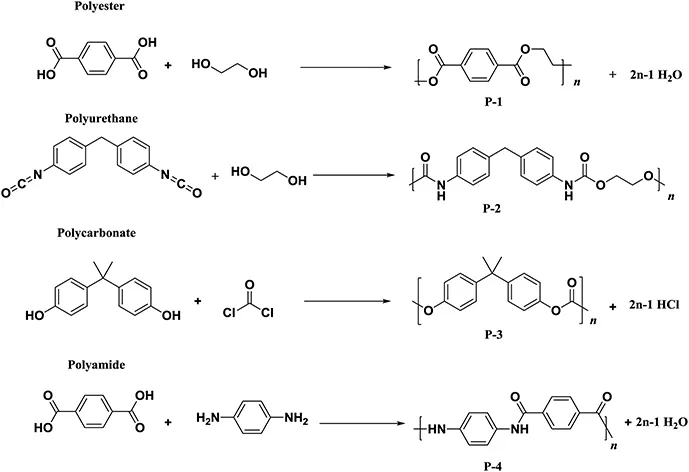
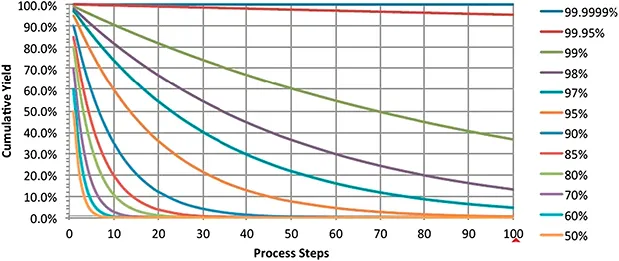
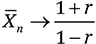
1.1.2 Accessibility and Capacity: Who is the Boss in the Market?
| Polymers | World production (million tons) |
| Polyesters | 71 in 2016 |
| Polyamides | 3.9 in 2011 |
| Polycarbonates | 5.1 in 2016 |
| Polyurethanes | 20 in 2015 |
Table of contents
- Cover
- Halftitle
- Series Editor
- Title
- Copyright
- Preface
- Contents
- Chapter 1 New Polymers From SuFEx Click Chemistry: Syntheses and Perspectives 1
- Chapter 2 Thiol Chemistry for Precision Polymer Synthesis 32
- Chapter 3 Precise Synthesis of Polyethylene-based Star Polymers: From Anionic Polymerization to Polyhomologation 65
- Chapter 4 Fabrication of Supramolecular Polymers 89
- Chapter 5 Olefin (Co)polymerizations Enabled by Catalyst Design Based on Sidearm Strategy 129
- Chapter 6 Bimetallic Complex Mediated Meso-epoxide Desymmetrization Copolymerization 167
- Chapter 7 Carbon Dioxide Copolymer From Delicate Metal Catalyst: New Structure Leading to Practical Performance 197
- Chapter 8 Chemosynthesis of Poly(ε-Lysine) via Ring-opening Polymerization of Cyclic Lysine 243
- Chapter 9 Fused (Hetero)Cyclic Polymers Synthesized by Alkyne-Based Polymerizations 264
- Chapter 10 Organocatalytic Synthesis of CO2(COS)-basedCopolymers 307
- Subject Index