![]()
1 The basics of mineralogy
1.1 What is a mineral?
‘In general terms, a mineral is an element or chemical compound that is normally crystalline and that has been formed as a result of geological processes’ – E.H. Nickel, writing on behalf of the International Mineralogical Association in the peer-reviewed journal Canadian Mineralogist in 1995.
Why use the caveat ‘general’? Well, there are just a few exceptions, even though virtually all minerals satisfy these criteria. One interesting exception is mercury. This familiar metal occurs in its elemental or native form in certain ore-deposits: its melting-point is –38.8 degrees Celsius, so that in most parts of the world it would occur in the liquid, rather than crystalline, state. However, it has formed as a result of geological processes and is regarded as a mineral. Likewise, there are a small number of naturally formed compounds that are amorphous, meaning that they are never found in a crystalline state: the copper carbonate georgeite is an example of an amorphous compound that is officially regarded as a mineral.
These few exceptions apart, we have a very straightforward definition to work with. In the same paper, the author goes on to make the distinction that compounds that have formed on materials of anthropogenic origin, such as metal-rich smelter-slags, are not to be regarded as minerals. Compounds that have formed on substrates of natural origin that have been exposed to geological processes by anthropogenic activities – such as tunneling or making road-cuttings, which could result in rocks being exposed to weathering agents – can be regarded as minerals.
At the time that this definition was published, there was a degree of dissent within the mineral collecting community, because the compounds occurring as a result of weathering of ancient smelter-slags often form beautiful, colourful crystals lining cavities in the slags. Some extensive collections of ‘slag-minerals’ have been built up by some collectors – hence their disappointment in reading that such specimens were no longer regarded as minerals. One suspects that this aspect of the definition of a mineral, and to what extent human activity should be included or excluded from Nature, will continue to prompt lively debate for years to come!
And what about the crystalline compounds and elements of extraterrestrial origin? Nickel goes on to state that extraterrestrial substances ‘were apparently produced by processes similar to those on Earth, and therefore such processes are now called geological, even though the term “geology” originally meant the study of rocks on this planet’. This seems reasonable enough. Terms often have to be adapted as circumstances change through the progress of science. So let us now further dissect the definition, firstly looking at what a chemical compound is and how it forms, and secondly, looking at crystals and crystal formation. We will look at the geological processes that lead to the formation of mineral deposits in later chapters; here we deal with the basics.
1.2 Elements and chemical compounds: a chemistry crash-course
Both elements and chemical compounds consist of the basic units of matter: atoms. An element, in its pure form, consists entirely of atoms of itself: thus pure gold consists entirely of atoms of gold. In nature, such purity is virtually unheard-of, so that the native elements will consist almost entirely of atoms of themselves, but will also include impurities – atoms of other elements. Compounds consist of atoms of two or more elements combined and held together by chemical bonds. So how does that work? To find out, we need to start with the basics: what are atoms, and how they may bond to one another to make cohesive substances?
Any atom consists of a dense central nucleus – making up over 99% of its admittedly tiny mass – which is surrounded by one or more negatively charged electrons. The nucleus consists of one or more protons, which carry a positive charge, and neutrons which, as the name suggests, are neutral, and with a similar mass to protons. The electromagnetic force between the positively charged protons and the negatively charged electrons (opposite charges strongly attract one another) is what binds the whole thing together.
Each chemical element has an atomic number which is defined by the number of protons in the nuclei of its constituent atoms. Thus hydrogen – element 1 – has a single proton in its nucleus; copper – element 29 – has 29 protons, and lead – element 82 – has 82 protons, and so on. The number of neutrons plus the number of protons gives a second value – the mass number. Because the number of neutrons can vary a little, some elements occur in nature as several varieties with different mass numbers. These varieties are known as isotopes. An example is carbon: it has three isotopes – carbon 12, 13 and 14. An atom of carbon 12 has six protons and six neutrons in its nucleus. It is by far the dominant isotope, comprising 98.89% of carbon in nature. Carbon 13, with six protons and seven neutrons, is stable but rare, making up 1.109% of the total. Carbon 14, also known as radiocarbon, is an unstable, radioactive isotope with six protons and eight neutrons, making up the extremely small remainder.
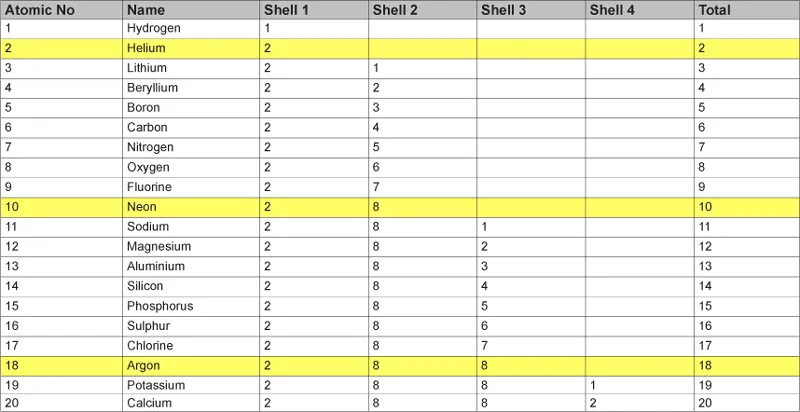
The electrons that surround the nucleus of an atom exist within a series of orbitals or shells, each being a zone of stable energy levels. Working outwards from the nucleus of an atom, the shells are labelled 1–6 and each holds a specific maximum number of electrons. Shell 1 can hold two electrons, shell 2 can accommodate eight; shell 3 eighteen, shell 4 thirty-two, and so on. For example, hydrogen, consisting of one proton and one electron, only has shell 1. Fluorine, with nine protons and nine electrons, has shells 1 and 2 – so there are two electrons in shell 1 and seven out of a possible eight in shell 2. Neon has ten protons and ten electrons, so that shells 1 (2 electrons) and 2 (8 electrons) are both filled. Sodium, on the other hand, has 11 protons and 11 electrons, with shells 1 and 2 filled with two and eight electrons respectively, but with a single electron in the succeeding shell 3. The configuration of sodium’s electrons may thus be written 2,8,1.
The table below shows the electron configuration of elements 1–20, demonstrating how successive shells fill up with increased atomic number:
Table 1.1 The electron configurations of the first twenty elements
Electrons may move up or down from one shell to another, but to do this they must either absorb or emit energy: those closest to the nucleus are held, because of their proximity, by the strongest electromagnetic force, so cannot easily be moved outwards. But for those electrons in the outermost shell, things are in most cases somewhat easier. This outermost shell is known as the valence shell: valence refers to the number of chemical bonds that an atom can form with other adjacent atoms, and it is controlled by the number of electrons and empty spaces in the valence shell.
A look at the Periodic Table of the elements (Table 1.2) shows that the elements are arranged in rows and columns, and that groups of chemically similar elements make up the columns – thus the halogens – fluorine, chlorine, bromine, iodine and astatine – occupy column 17 and the noble gases – helium, neon, argon, krypton, xenon and radon – make up column 18, and so on. This is no accident; the columns are set out to depict the number of electrons present in the outermost shell. So, the elements in column 1 (the alkali metals) have one outermost shell electron, column 2 elements (the alkaline earth metals) have two. Jumping momentarily past the transition metals, which occupy co...