
- 156 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Advanced Materials for Sodium Ion Storage
About this book
Globally, lithium ion batteries (LIBs) are leaders in the energy storage sector but there are concerns regarding load leveling of renewable energy sources as well as smart grids and limited availability of lithium resources resulting in cost increase. Therefore, sodium ion batteries (SIBs) are being researched as next-generation alternatives to LIBs due to their similar sustainability and electrochemistry. This book mainly focuses on the current research on electrode materials and proposes future directions for SIBs to meet the current challenges associated with the full cell aspect. Further, it provide insights into scientific and practical issues in the development of SIBs.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Advanced Materials for Sodium Ion Storage by Ranjusha Rajagopalan,Lei Zhang in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction for Sodium Ion Batteries
The depletion of fossil fuels increases the concern about environmental pollution. As a result, tremendous attention has been paid to novel renewable and cleaner energy sources, including wave, wind, and solar. Producing low cost and green renewable energy from these sources plays an important role in satisfying the growing demand for transportation fuel, heat, and electricity. Unfortunately, the intermittent ability of the renewable primary energy limits its effective practical application, which will result in instability of the power supply. From this point of view, the energy storage systems (EESs) are the most crucial aspects during the renewable energy production process, because the renewable energy can be stored gradually without concerns about its intermittent ability. According to the current understanding for energy production, it can be produced and stored with the help of different means, such as electrical, mechanical, electrochemical, or chemical means. Among these four different EESs technologies, it is promising to take advantage of the electrochemical secondary battery for large-scale storage of electricity because of its high flexibility to different conditions, relevant high energy conversion efficiency, and facial maintenance.
1.1 The History of the Sodium Ion Battery
The first commercialized electrochemical-based EES was the lithium ion battery (LIB) which was initially commercialized by Sony in 1991. LIBs quickly dominated the market of portable electronic devices and were identified as the most promising candidate to address the electrical grid concerns due to the high-specific storage capacity, long cycling life, and suitable working potential. However, with the increasing lithium demand within the last 10 years, the price of lithium compound is increased significantly due to the limited global lithium reserves. Worldwide concerns and tremendous attention have been paid to search for new electrochemical-based EESs.
Compared with LIBs, sodium ion batteries (SIBs) and potassium ion batteries (KIBs) have attracted great efforts in the past few years due to the practically unlimited nature of sodium and potassium resources. Unfortunately, potassium shows the largest ionic radius (~0.4 Å larger than Na+ and ~0.7 Å larger than Li+), leading to the limited choice of the available anodes and poor cycling stability of the electrodes in KIBs. Compared with Li and K, Na is the most abundant element on earth. In addition, a great number of Na-containing resources is available in the commercial market, which provides the opportunity for scalable electrode preparation. For example, 23 billion tons of soda ash is available in America alone. As a result, the cost for the SIBs electrodes are largely reduced when compared with LIBs, which makes SIBs more promising as the next generation EES and attracted tremendous research attention during the past few years.
Actually, the initial study of SIBs was started from the 1970s which is quite close to the first research about LIBs. Unfortunately, the following research on SIBs was abandoned after that because of the significant breakthrough in the successful commercialization of LIBs. With the rapid development of LIBs, the high cost and limited resource of Li contributed the transfer of the research focus from LIBs to SIBs. Exploring and developing effective electrodes and electrolytes for the SIBs now represent a hot research topic. Figure 1.1 shows the recent progress in SIBs.1
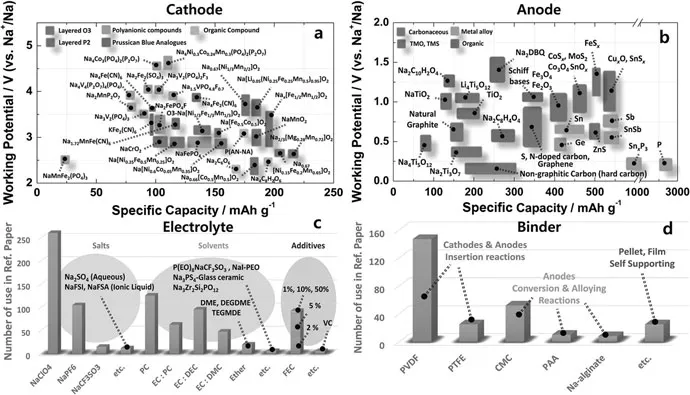
FIGURE 1.1
Recent research progress in sodium ion batteries: (a) cathode, (b) anode, (c) electrolyte, and (d) binder. (Copyright 2017, The Royal Society of Chemistry.)
Recent research progress in sodium ion batteries: (a) cathode, (b) anode, (c) electrolyte, and (d) binder. (Copyright 2017, The Royal Society of Chemistry.)
1.2 The Main Challenges for the Sodium Ion Batteries
SIBs and LIBs have similar components inside the systems, such as the anode, cathode, electrolyte, and separator (as shown in Figure 1.2).1 Furthermore, there is no significant electrical storage mechanism difference between SIBs and LIBs except for their ion carriers, which enable most of the electrode materials directly used from LIBs to SIBs. However, some differences still exist between LIBs and SIBs. For example, Na+ ions are 0.26 Å larger than the Li+ ions, leading to the relevant differences in the solid-electrolyte-interfaces (SEIs) formation, transport ability, and phase stability. In addition, Na+ ions have a higher atom mass and standard electrode potential (−2.71 V vs SHE for Na+ as compared to −3.02 V vs Standard Hydrogen Electrode (SHE) for Li+) than the Li+, leading to the lower energy density for SIBs.
1.2.1 Cathode Materials
According to the current development of SIBs, the overall ability of SIBs is determined by the performance of the cathode materials. Therefore, it is important to develop promising cathode materials with outstanding electrochemical abilities. Unfortunately, most attempts to further improve the cathodes have tackled the problem at the poor rate performance and the low specific capacity. For example, sluggish kinetics during sodiation/desodiation process and difficult transportation of the Na ions across the host-material framework happened due to the larger Na+ radius (0.98 Å) than Li+ (0.69 Å), resulting in the degradation in specific capacity and rate capacity. In addition, larger volume changes of the active materials will cause phase changes, resulting in deterioration in the cycling performance. More importantly, the low specific capacity of the most currently used cathodes limit the practical application of the full cells. Therefore, many recent research works have focused on efforts to overcome all these disadvantages by exploring and exploiting the potential properties of sodium-based cathodes.
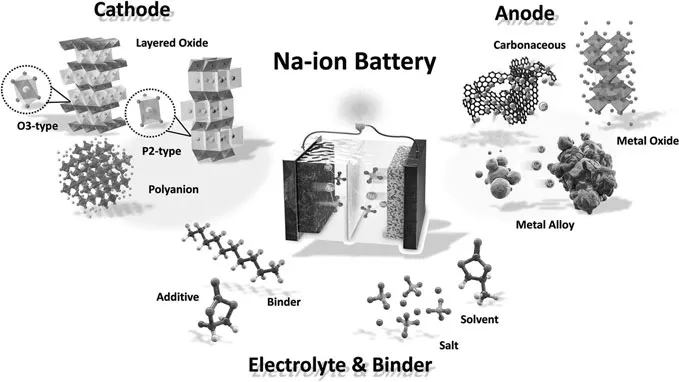
FIGURE 1.2
Illustration of a Na-ion battery system. (Copyright 2017, The Royal Society of Chemistry.)
Illustration of a Na-ion battery system. (Copyright 2017, The Royal Society of Chemistry.)
Four different kinds of materials can be used as promising cathode materials for SIBs, including transition materials, transition-metal fluorides, polyanion compounds, and organic materials. The transition materials, which mainly include layered sodium metal oxides and sodium-free metal oxides, were extensively studied during the 1970s and 1980s. The common molecule formula for these kinds of sodium metal oxides can be described as , where M is a transition metal. Apart from the transition materials, two main fluorides, such as the Perovskite transition-metal fluorides MF3 and sodium fluoroperovskites NaMF3 (M = Ni, Fe, Mn), can be treated as cathodes for SIBs. The stronger ionic bonding and electronegativity of fluorine than oxygen made these fluoride-based cathodes potential candidates for SIBs; especially due to their high operating voltages. Polyanionic phosphate compounds have been successfully used as cathode electrodes in LIBs. Therefore, it is interesting to explore the electrochemical ability of these polyanion phosphate and mixed polyanion materials as a cathode system in SIBs. After the relevant research during the past few years, these polyanionic systems are regarded as potential candidates for the practical SIBs due to their good structural stability, diversity, superior operating potential, thermal safety, high cycling life, and strong inductive phenomenon resulting from the highly electronegative anions. Different from the abovementioned inorganic materials, the organic compounds show unique abilities when used as cathodes in SIBs. For example, the organic electrodes are endowed with various advantages, such as structural diversity, less expensive, good safety, recyclable nature, and flexibility. Generally, two different kinds of organic materials are attracting the most research attention, these are metal organic materials and metal-free organic materials.
1.2.2 Anode Materials
Compared with the relevant research about cathode materials in SIBs, it is more difficult to find the appropriate anode candidates in SIBs. This is mainly because limited materials have been reported to be available as negative electrodes in SIBs. It can be seen that most of the previous reported cathode materials for LIBs can be used as cathode materials in SIBs just by replacing the lithium with sodium. Unfortunately, based on the currently promising anodes, such as graphite, which is a traditional commercial anode material in LIBs, most of them cannot be directly used as anodes in SIBs because SEI film generated on the surface of the active particles is unstable. As a result, the exploration for novel commercial anodes in SIBs is very important.
The current research about the anode materials for SIBs can be divided into four different categories based on the different electrochemical reaction mechanism between the anode materials and reversible Na+: the insertion materials, conversion materials, alloying materials, and organic materials. The carbon- and titanium-based materials are the most popular ones in the insertion materials, because the Na ions can be reversibly inserted/extracted into/from these kinds of materials due to their unique structural abilities. Conversion-type material, such as the transition metal sulfide, transition metal oxide, and transition metal phosphide, is another promising candidate for reversible energy storage, because it has high theoretical specific capacity for adopting Na+ during the conversion process. For the conversion materials, a new compound will be generated after the sodiation process, leading to the high reversible specific capacity. Alloying-type materials show promising electrochemical performance when they are employed as anodes for SIBs, because the alloying-type materials provide abundant active positions for Na ion insertion/extraction under a very low working voltage range. Various kinds of interactions can be conducted with Na ions, such as Sn, Bi, Si, Ge, As, Sb, and P. Compared with the abovementioned inorganic electrode materials, the organic ones give energy storage a new lease on life and provide benefits in terms of their mechanical flexibility, large stable structure changes, low density, low cost, environmentally friendly ability, and chemical diversity, which provide a promising application of organic materials as candidate for special batteries.
1.2.3 Electrolyte and Additions
As for the electrolyte and other additions, even more limited work has been conducted or published when compared with cathodes and anodes. The main reason for that is because the research work on the electrolyte and additions is more likely an interdisciplinary project which combines the research cooperation from the organic materials, inorganic materials, and electrochemical materials. As a result, poor research outcomes on these areas have been obtained during the past few years.
The practical application of the commercial full batteries is usually hindered by the decomposition of the liquid electrolytes under higher working voltage, leading to the lower energy densities of the full cells. Apart from that, the important SEI films are generated during the charging/discharging process. And the structural stability of this SEI film is mainly determined by the ingredients of the electrolytes, additions, and binders. Therefore, it is important to develop novel electrolytes and additions to satisfy the requirements from the future energy storage systems.
Some necessary requirements and key points of the electrolyte are needed for the practical application of the SIBs, including the chemical inertness, low cost, scalable production available, electrical insulating and ionically conductive, environmentally friendly, and facial fabrication process. For the liquid electrolyte, the polar ability should be well maintained even under high dielectric constant. In addition, it should have a low viscosity to enhance the transfer of ions. As for the additive inside the electrolyte, it is required to generate the stable SEI films between the electrolyte and electrode. Moreover, the safety of the full cells and the electrochemical stability of the electrolytes should be improved with the help of using extra additives. But for the binders, the most important role is to maintain the structural stability of the electrodes during the cycling process. For example, because of the large volume changes of most anode materials, a suitable binder could effectively address this problem via the strong chemical bonding between binder and electrode, leading to improved structural stability. Furthermore, a tight contact between the electrode materials and the current collector can also be obtained with the introduction of the binders, which can significantly avoid the stripping of the active materials from the current collector.
In summary, it can found that the research about the SIBs is relatively limited and more efforts need to be made to further improve its electrochemical ability. Therefore, in this book, we will emphasize the current research development of the cathodes, anodes, and electrolytes of the SIBs to give an overall understanding for the SIBs.
Reference
1. Hwang, J. Y., Myung, S. T., Sun, Y. K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46 (12), 3529–3614.
2
Electrochemical Reaction Mechanism in Sodium Ion Batteries
2.1 Introduction
The modern world is getting more and more dependent on energy to have an uninterrupted power supply for t...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Acknowledgments
- Authors
- 1. Introduction for Sodium Ion Batteries
- 2. Electrochemical Reaction Mechanism in Sodium Ion Batteries
- 3. Anode Materials for Sodium Ion Batteries
- 4. Cathode Materials for Sodium Ion Batteries
- 5. Electrolytes, Additives, and Binders for Sodium Ion Batteries
- 6. Current Challenges and Future Perspectives
- Index