![]()
Chapter One
Nickel Superalloys
INTRODUCTION
This chapter focuses on casting alloys. Two groups of alloys are considered: single-crystal and polycrystalline state of nickel superalloys and Ni3Al single-crystal intermetallic alloys. All of these alloys are used for manufacturing of gas turbine blades. Results of the study on the influence of severe deformations on the structure and properties of the nickel superalloys are presented in this chapter. Nickel superalloys used in aircrafts operate at the highest levels of stresses and temperatures (up to 1200°C). Also, nickel superalloys used in electric power stations operate at lower temperatures (800-900°C), because of that the nickel gas turbine blades may also be under extreme stresses when the turbine power increases. The main factor for a long trouble-free operation time of the turbine blades is their structural and phase stability at high temperatures. In this chapter, we present the results of the analysis of the gas turbine blade failure under external effects (high temperature, rotation, vibration). We also found that a magnetic non-destructive testing of the structure state of the working nickel gas turbine blade may be used in the industrial monitoring process of its operation, maintenance and failure.
1.1 Structure, Mechanical, and Magnetic Properties of Ni3Al-Based Alloys
1.1.1 Effect of alloying elements on Ni3Al
Ni3Al intermetallic compound (γ′-phase) is the main strengthening phase of the modern heat-resistant nickel superalloys used in the aircraft technology and stationary power gas turbine equipment. Ni3Al intermetallic compound (L12, Pm3m) exists within a narrow concentration interval which is close to 75 at.% Ni. The aluminum atoms occupy the corners and the nickel atoms are face-centered in the Ni3Al crystal lattice.
The main feature of Ni3Al is its ability to dissolve almost all transition elements. This maintains a high degree of long-range ordering and L12 ordered structure type with two sublattice (nickel and aluminum). Numerous experimental data, reviewed by Sluiter and Kawazoe (1995), allow us to assert that the atoms of Nb, Ti, V, and W mainly replace the positions of aluminum and Co atoms that are included in the nickel sublattice. Fe and Cr atoms can equally be found in both sublattices.
In this section, we discuss the influence of alloying elements on the properties of the Ni3Al intermetallic compound.
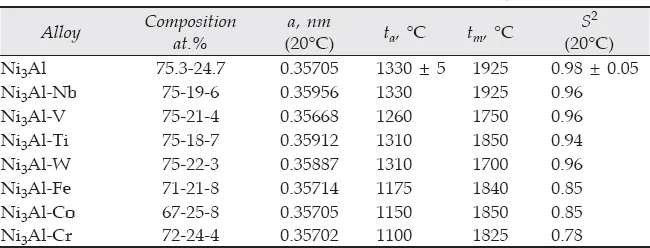
Lattice parameter. In the literature, the Ni3Al stoichiometric state has different values for the lattice parameter a, ranging from 0.3566 ± 0.0001 nm (Stoeckinger and Neumann 1970) to 0.3589 nm (Stoloff 1989). It is known (Stoloff 1989, Cahn et al. 1987b) that the value of the lattice parameter of Ni3Al in cast alloy can be unstable and changes during long-term exposure even at room temperature. In Stepanova et al. (2000), the change of the Ni3Al lattice parameter on alloying with different chemical elements was studied by X-ray at room temperature and under continuous heating of the sample in a diffractometer chamber. The chamber was evacuated and the homogenized single crystal <001> samples were used for the study. Solution treatment (1578 K-2 hours) and two stage aging processes (1373 K-4 hours, vacuum cooling and 1113 K-20 hours, air cooling) of the samples, as it is shown by Stepanova et al. (2000), eliminates liquation and strain arising during the growth of a single crystal. In this case, the diffraction lines become narrower; for example, for all investigated alloys the (004) diffraction line width at half height was reduced by 28-35% after annealing. Room temperature values of the crystal parameter a (after annealing of the alloys studied) are given in Tables 1.1 and 1.2 (Stepanova et al. 2000). The summary in the study of the lattice parameter change for doped Ni3Al by Stepanova et al. (2000) coincides with the conclusions obtained in the study of complex-alloying nickel superalloys. These conclusions may be summarized as follows: an introduction of the Nb and Ti atoms into the Ni3Al crystal lattice leads to increasing values of the crystal parameter a; alloying with V atoms reduces the lattice parameter of Ni3Al. The Fe, Cr, and Co atoms have a little effect on the values of the Ni3Al crystal parameter (Mishima et al. 1985).
X-ray studies during continuous heating were done in the temperature range from room temperature to 1200°C under vacuum conditions. The heating rate was 5°C/min. An increase of the lattice parameter a values with increasing temperatures was found in almost all studied ternary alloys, with one exception (Tables 1.1 and 1.2) Savin et al. 2000a, 2000b.
Table 1.1 Parameters of the investigated alloys
The coefficients α of thermal expansion of alloys based on Ni3Al were experimentally determined by Stepanova et al. (2000). Table 1.2 presents two temperature ranges for the coefficients α.
Table 1.2 Parameters of the series of ternary Ni3Al-based alloys
Alloy | α·105, deg–1, (20-1000°C) | α·105, deg–1, (1000-1200°C) |
Ni3Al | 1.51 ± 0.04 | 1.91 ± 0.04 |
Ni3Al-Nb | 1.46 | 1.92 |
Ni3Al-V | 1.45 | 1.83 |
Ni3Al-Ti | 1.30 | 1.84 |
Ni3Al-Fe | 1.77 | 2.10 |
Ni3Al-Co | 1.77 | 1.59 |
Ni3Al-Cr | 1.63 | 2.41 |
In the range of 20-1000°C, the α values are similar to each other in alloys Ni3Al-X, where X = {Nb, V, Ti}, as well as for the binary Ni3Al alloy. In the alloys Ni3Al-X (X={Fe, Co, Cr}), the coefficient α increases up to 1.77 · 10–5 deg–1. In the temperature range of 1000-1200°C, the values of the α coefficient of thermal expansion increase in all alloys except the alloy doped with cobalt, in which the coefficient α has a value of 1.59·10–5 deg–1.
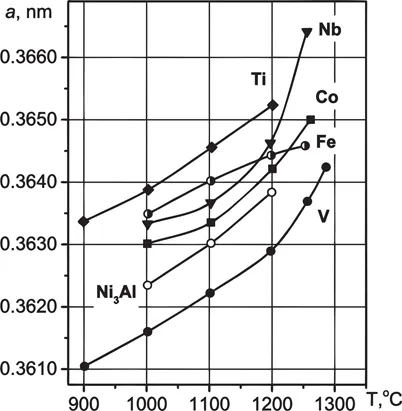
In the Ni3Al-V alloy, the value of the crystal parameter α is smaller than for Ni3Al over the entire investigated temperature range. The changes of the lattice parameter with increasing temperature are shown in Fig. 1.1. The temperature dependence of the α coefficient of thermal expansion may be explained by the atomic substitution process in the Ni3Al crystal lattice.
Fig. 1.1 Temperature dependence of the lattice parameter for Ni3Al and the series of Ni3Al-based ternary alloys doping with Nb, Ti, Co, Fe, V (Stepanova et al. 2000).
It was theoretically suggested by Enomoto and Harada (1989) that the temperature dependence of replacement type occurs in ternary alloys based on the elements that are placed at room temperature in both sublattices (in this case, chromium and iron). With increasing temperature, the atoms of these alloying elements start to replace mainly the positions of the aluminum atoms and, therefore, the vacancies appear in the nickel sublattice. The number of the nickel vacancies increases with increasing temperature. Work done by Stepanova et al. (2000) experimentally confirms correctness of the Enomoto’s calculations. The alloy doped with cobalt (atoms of which are placed in the nickel sublattice) has different values of the α coefficient.
All these results suggest that the alloying elements can be divided into three groups according to the type of substitution: 1) Nb, V, Ti; 2) Fe, Cr; and 3) Co.
Stoloff (1989) gives the value α= 1.51·10–5 deg–1 at 800°C. According to Stoeckinger and Neumann (1970), the coefficient of thermal expansion α in binary Ni3Al alloy can be estimated as (1.54 ± 0.04) · 10–5 deg–1 for temperatures up to 1000°C and (2.03 ± 0.04) · 10–5 deg–1 at temperatures above 1000°C. These values are in a good agreement with the above-mentioned results.
Degree of long-range order. From this point of view, the concept of “degree of long-range order” with respect to intermetallic compounds makes no sense, although it is, of course, can be defined formally by X-ray data. It will always be close to one (Stoeckinger and Neumann 1970) or 0.98 (Pope and Garin 1977).
The nature of the interatomic interactions in the intermetallic compounds is much closer to the pure chemical compounds tha...