
Gas Injection into Geological Formations and Related Topics
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Gas Injection into Geological Formations and Related Topics
About this book
This is the eighth volume in the series, Advances in Natural Gas Engineering, focusing on gas injection into geological formations and other related topics, very important areas of natural gas engineering. This volume includes information for both upstream and downstream operations, including chapters detailing the most cutting-edge techniques in acid gas injection, carbon capture, chemical and thermodynamic models, and much more.
Written by some of the most well-known and respected chemical and process engineers working with natural gas today, the chapters in this important volume represent the most state-of-the-art processes and operations being used in the field. Not available anywhere else, this volume is a must-have for any chemical engineer, chemist, or process engineer in the industry. Advances in Natural Gas Engineering is an ongoing series of books meant to form the basis for the working library of any engineer working in natural gas today.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Modifying Effects of Hydrogen Sulfide When Contemplating Subsurface Injection of Sulfur
AbstractTransportation and handling of molten sulfur is inevitably challenging due to the anomalous rheological behaviour of sulfur with changing temperature. After melting at 115 °C, sulfur’s viscosity remains close to 10 cP. The onset of the λ-transition region is observed at T ≈ 160 °C. The viscosity drastically rises in this region, increasing to a maximum of about 93000 cP at 187 °C. This anomaly is due to the cleavage of sulfur rings and production of reactive diradical sulfur species that concatenate to create sulfur polymers. The entanglement of these sulfur chains causes the dramatic rise in viscosity. Within this work, new data is reported that examines the modifying effects of hydrogen sulfide (H2S) within liquid sulfur. This may be used when considering the potential injection of liquid sulfur into depleted reservoirs for safe long-term storage. H2S, when present in liquid sulfur, can either physically dissolve or chemically react to generate polysulfane. The chemical reaction causes significant changes in the sulfur chain distribution and consequently changes the viscosity curve of liquid sulfur as a function of temperature. This study reports viscosity measurements performed from 120 < T < 280 °C with concentrations of H2S in sulfur ranging from 0 ppmw to 284 ppmw.Keywords: Rheology, viscosity, λ-transition, sulfur, polymers, hydrogen sulfide
1.1 Introduction
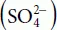
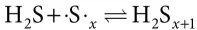
1.2 Experimental
1.2.1 Materials
Table of contents
- Cover
- Table of Contents
- Preface
- 1 Modifying Effects of Hydrogen Sulfide When Contemplating Subsurface Injection of Sulfur
- 2 Experimental Determination of CO2 Solubility in Brines At High Temperatures and High Pressures and Induced Corrosion of Materials in Geothermal Equipment
- 3 Experimental Study of the Liquid Vapour Equilibrium of the System Water-CO2-O2-NOx Under Pressure at 298 K
- 4 The Use of IR Spectroscopy to Follow the Absorption of CO2 in Amine Media – Evaluation of the Speciation with Time
- 5 Solubility of Methane, Nitrogen, Hydrogen Sulfide and Carbon Dioxide in Mixtures of Dimethyl Ethers of Polyethylene Glycol
- 6 Water Content of Hydrogen Sulfide – A Review
- 7 Acid Gas Injection at SemCAMS Kaybob Amalgamated (KA) Gas Plant Operational Design Considerations
- 8 Reciprocating Compressors in Acid Gas Service
- 9 Case Study: Wellbore Thermodynamic Analysis of Erhao Acid Gas Injection Project
- 10 Selecting CO2 Sinks CCUS Deployment in South Mid-West Kansas
- 11 Salt Precipitation at an Active CO2 Injection Site
- 12 The Development Features and Cost Analysis of CCUS Industry in China
- 13 CO2 Movement Monitoring and Verification in a Fractured Mississippian Carbonate Reservoir during EOR at Wellington Field in South Kansas
- 14 Simulation Study On Carbon Dioxide Enhanced Oil Recovery
- 15 Blowout Recovery for Acid Gas Injection Wells
- 16 The Comprehensive Considerations of Leak Detection Solutions for Acid Gas Injection Pipelines
- 17 Injection of Non-Condensable Gas in SAGD Using Modified Well Configurations - A Simulation Study
- 18 The Study on the Gas Override Phenomenon in Condensate Gas Reservoir
- 19 Study on Characteristics of Water-Gas Flow in Tight Gas Reservoir with High Water Saturation
- 20 The Description and Modeling of Gas Override in Condensate Gas Reservoir
- 21 Research on the Movable Water in the Pores of Tight Sandstone Gas Reservoirs
- 22 Probabilistic Petroleum Portfolio Options Evaluation Model (POEM)
- Index
- Also of Interest
- End User License Agreement