
eBook - ePub
Organoselenium Chemistry
- 436 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Organoselenium Chemistry
About this book
Organoselenium Chemistry is a unique resource in this branch of organic/organometallic chemistry. The authors give an overview of synthesis strategies, introduce bioactive and environmentally friendly organoselenium compounds and discuss their applications from organic synthesis to the clinic.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Organoselenium Chemistry by Brindaban C. Ranu, Bubun Banerjee, Brindaban C. Ranu,Bubun Banerjee in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1 Synthesis of Organoselenides by Coupling Reaction and C–H Activation – Recent Advances
Brindaban C. Ranu
School of Chemical Sciences, Indian Association for the Cultivation of Science, Kolkata, India
Tubai Ghosh
School of Chemical Sciences, Indian Association for the Cultivation of Science, Kolkata, India
Subir Panja
School of Chemical Sciences, Indian Association for the Cultivation of Science, Kolkata, India
Swapnadeep Jalal
School of Chemical Sciences, Indian Association for the Cultivation of Science, Kolkata, India
1.1 Introduction
The area dealing with the synthesis of organoselenides has experienced a tremendous growth in last two decades. A number of methods have been reported. This chapter highlights the recent reports on various methods for the synthesis of selenides by cross-coupling and C–H activation. The metal-catalyzed as well as metal-free procedures have been discussed. Besides Pd, the catalysis by other metals such as Cu, Ni, Ru and Ca have also been addressed. Under nonmetallic catalysis I2, (TBAI) and hypophosphorus acid (H3PO2) have been discussed.
Among the several organochalcogenides, organoselenium compounds are of considerable interest as selenides are found useful in the areas of agrochemicals, insecticides, and drugs (Figure 1.1) [1, 2, 3, 4, 5, 6]. Apart from biological applications, they are also used as useful intermediates and catalysts in several organic transformations [7, 8, 9]. They also show wide variety of applications as functional materials [10]. A large number of methods have been developed for the construction of C–Se bond both under metal free as well as in the presence of transition metal.
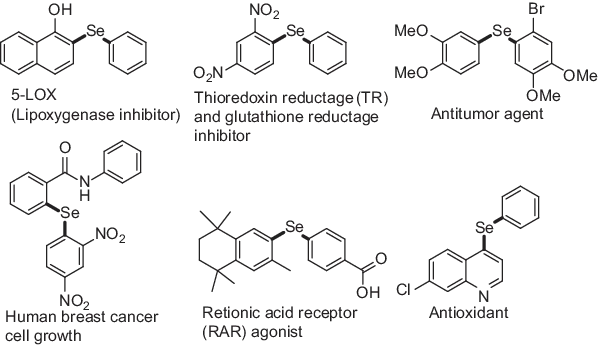
Figure 1.1: Examples of biologically active organoselenides.
This chapter covers the synthesis of organoselenium compounds through C–H activation and cross-coupling reactions.
1.2 Reactions involving C–H activation
In recent years, transition metal-catalyzed/mediated C–H bond functionlization became an important tool for the synthesis of organic molecules [11, 12, 13, 14, 15]. The use of C–H bonds toward transformable functional group is advantageous because C–H bonds are the most abundant moieties in organic molecules. Thus, one-step conversion of these C–H bonds to the desired functionality minimizes the synthetic pathways, saves reagents and reduces waste of solvents and time.
In this context, Nishihara and coworkers reported the synthesis of benzoisoselenazolone and its derivatives by nickel-catalyzed dehydrogenative direct selenation of C(sp2)–H bonds with elemental selenium powder under aerobic condition (Scheme 1.1) [16]. After optimization it was observed that a combination of 10 mol % of Ni(OAc)2·4H2O, 20 mol % of PPh3, 2 equiv. of Na2CO3 and 3 equiv. of n-Bu4NCl along with 2 equiv. of selenium powder in DMF at 120 °C under air provided the best results among others. A range of functional groups including both electron-donating and electron-withdrawing substituents were compatible under the optimized oxidative conditions and the corresponding benzoisoselenazolone derivatives were formed in good-to-excellent yields. Apart from benzamides, acrylamides were also found to be suitable for the desired transformations. The newly formed benzoisoselenazolone derivatives were readily converted into a variety of useful organoselenium compounds. Based on the control experiments, the author proposed a mechanistic cycle that the reaction proceeds by the formation of nickelacycle(II) and single-electron oxidation of the stable nickelacycle(II) species under aerobic condition.
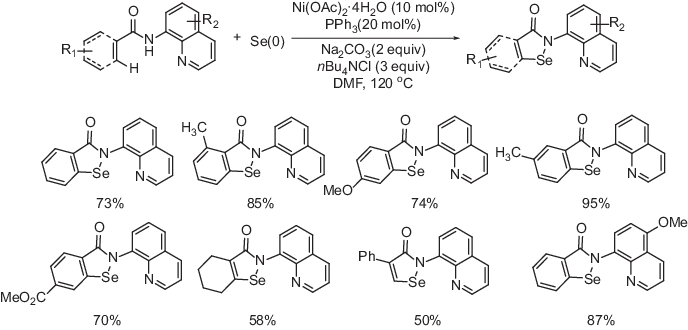
Scheme 1.1: Nickel-catalyzed dehydrogenative direct selenation of C(sp2)–H bonds.
Synthesis of unsymmetrical ferrocene aryl chalcogenides by C–H activation of ferrocene amide using 8-aminoquinoline as a directing group has been developed by Sattar et al (Scheme 1.2) [17]. The reaction occurs in the presence of copper(II) salt as the active catalyst, silver acetate as an oxidant at 80 °C in DMSO. A range of diaryl diselenides containing electron-deficient and electron-withdrawing substituents underwent successful coupling with ferroceneamide under the optimized reaction conditions and the bis-arylselenylated ferrocenes were obtained. Although the reactions successfully produced the desired arylselenylated ferrocene using diaryl diselenides, dialkyl diselenides failed to initiate the reaction. The reaction went smoothly with other diaryl dichalcogenides too.
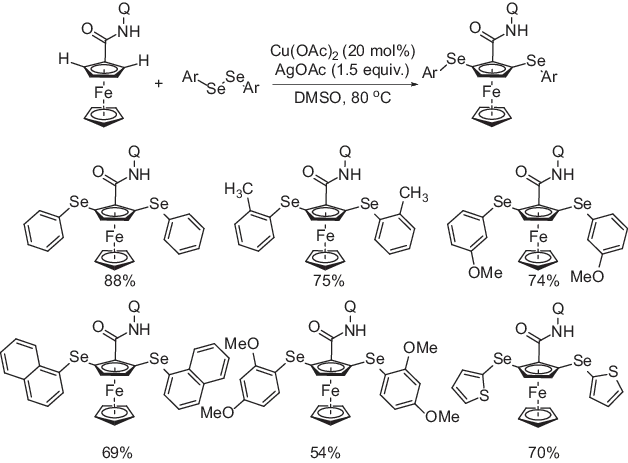
Scheme 1.2: Copper-catalyzed 8-aminoquinoline-assisted aryl selenylation of ferrocene amide.
C-4 Selenylated isoquinolin1(2H)-one was synthesized using a new and facile AgSbF6-mediated procedure through a radical pathway (Scheme 1.3) [18]. The reaction occurs in the presence of equimolar amount of isoquinolin-1(2H)-ones and diaryl diselenide using AgSbF6 as an oxidant in dichloroethane under reflux for 8 h. Among several other commonly used oxidizing agents, AgSbF6 was found to be the most effective one. The reaction shows excellent regioselectivity and broad substrate scope. The corresponding C-4 selenylated derivatives were obtained in excellent yield. Apart from isoquinoline moiety, the reaction occurs without any difficulty when pyridin-2(1H)-one was used.
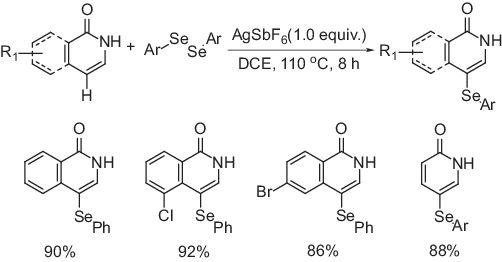
Scheme 1.3: AgSbF6-mediated C-4 selenylation of isoquinolin-1(2H)-ones.
As recently reported by Ranu and his coworkers, when 2-naphthol and its derivatives were allowed to react with styrenyl selenocyanate/diaryl diselenide in the presence of a base at room temperature, selenylation occurs selectively at the 1-position of 2-naphthol unit (Scheme 1.4) [19]. The reaction occurs in the presence of Cs2CO3 as a base in DMSO solvent at room temperature in the absence of any transition metal or oxidants. Both electron-donating and electron-withdrawing styrenyl selenocyanate reacted with 2-naphthol and its derivatives at room temperature and the corresponding 1-styrenyl-selenylated naphthol derivatives were obtained in good yields (Scheme 1.4a). The electronically diverse diaryl diselenides also provided the desired 1-arylated derivatives without any difficulty (Scheme 1.4b). The reactions are relatively fast (2−4 h) and high yielding. The reaction is also feasible in gram scale. More importantly, the unaffected hydroxyl group (–OH) can be further functionalized. Initially, the base Cs2CO3 reacts with 2-naphthol to form naphtholate anion intermediate (A) (Scheme 1.4c), which reacts with styrenyl selenocyanate/diryl diselenide at the 1-position to form the species (B). The intermediate (B) then undergoes aromatization via proton elimination to form the desired 2-selenylated product.
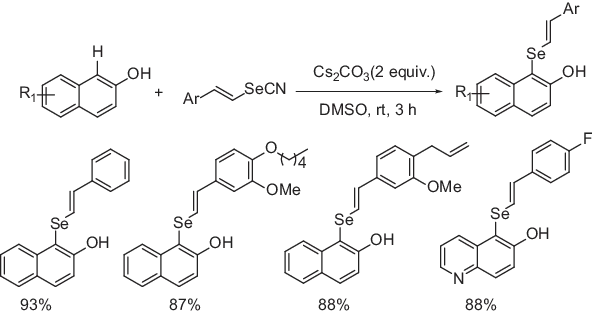
Scheme 1.4a: Transition ...
Table of contents
- Title Page
- Copyright
- Contents
- 1 Synthesis of Organoselenides by Coupling Reaction and C–H Activation – Recent Advances
- 2 Synthesis of Organoselenium Scaffolds through Selenium Radical Formation
- 3 Role of Isoselenocyanates for the Synthesis of Selenium-Containing Heterocycles
- 4 Selenoureas and Their Applications
- 5 Selenium Compounds as Reagents, Catalysts, and Ligands
- 6 Synthesis of organoselenium compounds using nonconventional reaction media
- 7 Synthesis and Biological Activity of Five- and Six-Membered Se-Containing Heterocycles
- 8 Chemistry and pharmacology of synthetic organoselenium compounds
- 9 Selenoamides, selenazadienes, and selenocarbonyls in organic synthesis
- 10 Understanding the Chemistry of Selenoenzymes by Synthetic Organoselenium Compounds
- Index