
- 399 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Organophosphorus Pesticides
About this book
In order to get a general concept of organophosphorus pesticides with such a variety in structure and biological activities, consideration of each aspect of chemistry, biochemistry, and the applied sciences is necessary. This book consists of these three main parts. After the presentation of the background of phosphorus chemistry in Chapter 1, stress was put on the chemical and biochemical reactions of organophosphorus pesticides, including synthesis, analysis, metabolism mode of action, and other interesting aspects in Chapter 2 to 4, and on the structure-pesticidal activity relationship in Chapter 5.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Organophosphorus Pesticides by Morifusa Eto in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter I
INTRODUCTION
A. History
Organic compounds of phosphorus are the essential constituent of protoplasm and play important roles for maintenance of life, for example, as nucleic acids, nucleotide coenzymes, metabolic intermediates, and phosphatides. On the other hand, many organophosphorus compounds are artificially produced for the practical uses of lubricants, oil additives, plasticizers, and pesticides.1 Organophosphorus pesticides include not only insecticides, but also fungicides, herbicides, and others. It is surprising to know that such great varieties in chemical, physical, and biological properties are governed by the selection of groups attached on the phosphorus atom.
The research in the field of the organic chemistry of phosphorus was first undertaken by Lassaigne, in 1820, to prepare phosphate esters.2 The chemistry of organophosphorus compounds was developed extensively by Michaelis in Germany, during the late 19th century and the beginning of this century.3 He performed many works and gave a foundation for this field, particularly on the chemistry of compounds containing the P-N bond.4 Overlapping the latter stages of Michaelis, a Russian chemist, A. E. Arbuzov, conducted extensive research, especially on the chemistry of trivalent phosphorus compounds, including the famous Michaelis-Arbuzov reaction to form the P-C linkage.5 His work has been continued by his son, B. A. Arbuzov.
On the other hand, since Harden and Young disclosed, in 1905, the importance of inorganic phosphate on alcoholic fermentation and discovered fructose diphosphate as a metabolic intermediate,6 many interesting organic phosphate esters have been found from biological sources. Since 1945, a systematic investigation on phosphorylation reactions has been carried out by the school of Sir Todd, and then by Cramer, in order to synthesize naturally occurring phosphate esters.7,8
Physiologically abnormal effects of organophosphorus compounds were first observed in dialkyl phosphorofluoridates by Lange and Krueger in 1932.9 They intended to find new types of organic pesticides at that time.10 During the Second War, Saunders in England and Schrader in Germany worked on toxic phosphorus compounds. Saunders synthesized nerve poisons, including diisopropyl phosphorofluoridate (DFP).11 Schrader and his co-workers found, in 1937, a contact insecticidal activity in some organophosphorus compounds of the general formula:
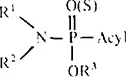
where R1, R2, and R3 are alkyl groups, and “acyl” is an inorganic or organic acid radical such as Cl, F, SCN, and CH3COO.’ 2 Since this point, a variety of fruitful results have been brought forth. Schrader et al. found, in 1941, a systemic insecticide, octamethylpyrophosphoramide (OMPA), which was later named schradan after its discoverer, and also discovered a number of insecticidal organophosphorus esters, including the first practical insecticide named “Bladan,” which contained tetraethyl pyrophosphate (TEPP) and was marketed in Germany in 1944. The synthesis of tetraethyl pyrophosphate was first performed by Moschnine, and then by De Clemont in 1854, and was repeated by several authors, including Nylén (1930) and Arbuzov (1938), without their taking notice of its toxicity. De Clemont tasted TEPP, but did not realize its toxicity.10
The great advancements in agricultural practice and scientific knowledge on the structure-activity relationship of organophosphorus insecticides were achieved by the discovery of compound No. 605, named parathion, diethyl p-nitrophenyl phosphorothionate, by Schrader in 1944. Although parathion itself is extremely toxic to mammals as well as to insects, many less toxic insecticides have been developed by slight structural modifications; for instance, chlorthion,13 fenthion,14 and fenitrothion15 were discovered in 1952, 1958, and 1959, respectively.
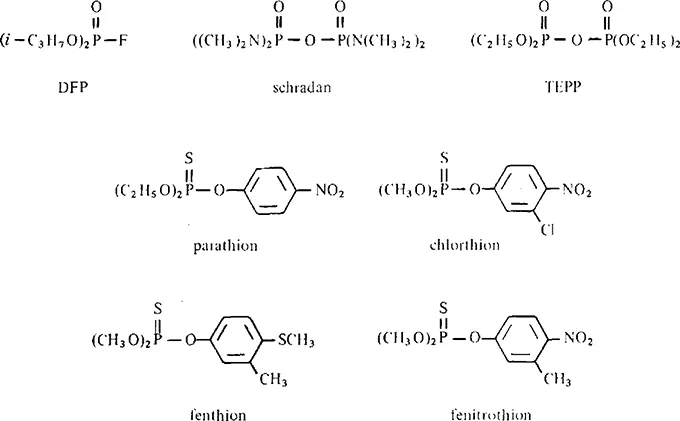
All these active compounds have an acid anhydride linkage and a general formula for biologically active organophosphorus compounds first proposed by Schrader:12
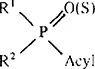
where, R1 and R2 = alkyl, alkoxy or amino groups, and acyl = any acid residue.
Another important compound with low mammalian toxicity, malathion, discovered by American Cyanamid Co. in 1950, has the carboxy ester group. Demeton, found in 1951 by Bayer AG, and it...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Foreword
- Preface
- The Author
- Table of Contents
- Chapter I Introduction
- Chapter II Synthesis
- Chapter III Chemical Reactions
- Chapter IV Biochemistry
- Chapter V Individual Pesticides
- References
- Index