
- 137 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The intention was to produce a book which perforce would never be far from the laboratory, although CRC's use of Handbook in another connection precludes our use of that word in the title.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Gas-Sensing Probes
M. Riley
TABLE OF CONTENTS
I. Introduction
II. Classification
A. General
B. Membrane Materials
III. Functional Details
A. Response Mechanism
B. Response Range
C. Selectivity
D. Response Time
E. Temperature Effects
F. Osmotic Effects
IV. Practical Usage
A. Method Development
1. Sample Pretreatment
2. Standardization
B. Manual Analysis
C. Continuous Flow Analysis
1. Laboratory Analysis
2. On-Line Monitoring
D. Gas Analysis
V. Specific Applications Of Gas-Sensing Probes
A. Ammonia Probe
B. Sulfur Dioxide Probe
C. Carbon Dioxide Probe
D. Nitrogen Oxide Probe
E. Other Probes
References
I. Introduction
It is now more than 8 years since the appearance of the first commercial potentiometric gas sensor for ammonia. Similar sensors for other gases subsequently appeared, but the ammonia gas-sensing probe remains the most significant in terms of sales volume and now ranks alongside the best-selling ion-selective electrodes in this respect. These gas-sensing probes display outstanding selectivity by comparison with many ionselective electrodes and are not restricted to the determination of gases; they are widely used for the determination of ions which can be converted to gases by appropriate chemical pretreatment, ammonium ion, for example, being determined as ammonia after making the solution alkaline and nitrite ion being determined as oxides of nitrogen after making the solution acidic.
In 1957, Stow et al.1 first described the concept of determining the partial pressure of gas dissolved in a sample by measuring the pH of a thin film of solution separated from the sample by a hydrophobic, gas-permeable membrane. They constructed a probe to measure the partial pressure of carbon dioxide in blood, which was subsequently improved by Severinghaus and Bradley,2 who considered the theoretical principles in more detail. About 10 years later, the previous work was reviewed by Severinghaus,3 who reported the effects of various membrane materials and internal electrolytes, and by Smith and Hahn.4
Despite the widespread use of the so-called “Severinghaus” electrode in clinical medicine, little work was done to extend the principle to the determination of other gases. The situation might have been different if there had been a specific requirement for such determination in clinical practice, although it is now clear that the lack of the microporous synthetic polymers, suitable for use as membrane materials, which have become available only relatively recently, was at least a contributory factor in delaying progress. In any event, more than a decade elapsed before the next significant advance occurred, and probes for other acidic and basic gases began to make an appearance.
In 1973 Ross et al.5 described several gas-sensing probes and their mode of operation and suggested several others which might be feasible. Bailey6 has provided a comprehensive review of the field up to 1976; since then, no radical new developments have occurred, but the area of application of gas-sensing probes has been extended to the solution of a wider variety of analytical problems.
II Classification
A. General
Strictly, these sensors are neither ion selective nor are they electrodes. They comprise an ion-selective electrode in combination with a suitable reference electrode to form a complete electrochemical cell, whose e.m.f. (electromotive force) is a function of the activity of the determinand gas in the sample. However, because it is the ionic activity of one of the components of the thin film of electrolyte which is directly measured, they are properly considered as part of the family of potentiometric ion-selective electrodes. Nevertheless, the name “gas-sensing probes” has been proposed7 for these sensors in an attempt to resolve the nomenclature difficulty, and this name will be used here.
Two distinct types of gas-sensing probes are now in use; all the initial developments in the field were concerned with gas-sensing membrane probes, while the somewhat different gas-sensing probes without membranes appeared later.
The construction of a typical gas-sensing membrane probe is illustrated in Figure 1. This shows a probe based on a glass electrode with a slightly convex pH-sensitive tip, which is held against the gas-permeable membrane so as to sandwich a thin film of the internal electrolyte between itself and the membrane. On immersion of the probe in a sample, determinand gas diffuses through the membrane until the partial pressure of the gas in the thin film of electrolyte is equal to that in the sample. This equilibrium partial pressure of determinand gas determines the pH in the thin film, which is measured by means of the glass electrode and a suitable reference eletrode (commonly a silver/silver halide electrode) immersed in the bulk of the internal electrolyte.
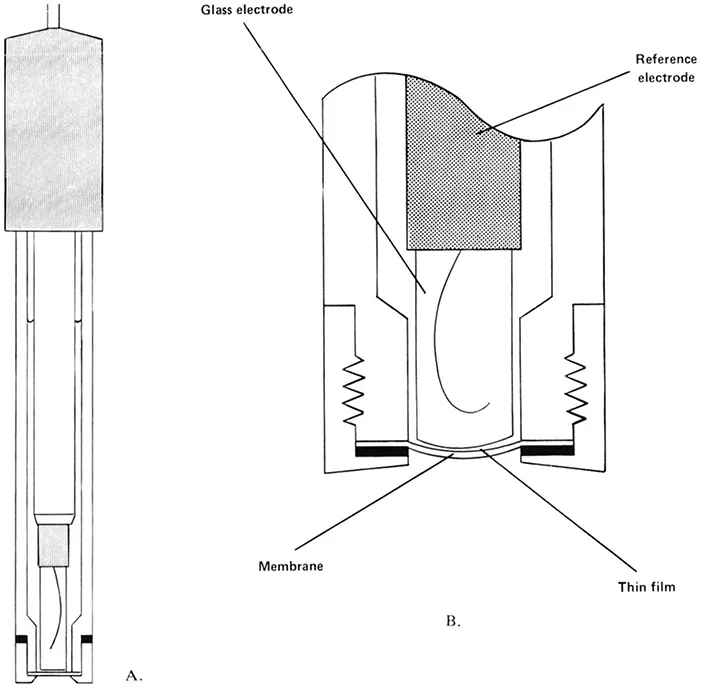
FIGURE 1. Construction of a typical gas-sensing membrane probe; A — overall layout and B — enlarged cross-section of sensing tip.
Gas-sensing probes without membranes, otherwise known as “air-gap electrodes”,8 do not differ significantly in principle from gas-sensing membrane probes. An air gap several millimeters in thickness replaces the membrane, and the probe is suspended above the surface of the sample, which is contained in a special sealed vessel; equilibration between the sample and the thin film occurs by diffusion of determinant gas through the air gap. The thin film of electrolyte is applied, normally with a special sponge, to the pH-sensing membrane of the glass electrode prior to measurement; the electrolyte incorporates a suitable wetting agent to stabilize the film.
The relative merits of gas-sensing membrane probes and gas-sensing probes without membranes have been discussed previously,7 and several benefits conferred by a membrane were enumerated. The arguments may be summarized by stating that gas-sensing membrane probes are, in general, more useful because they are easier to use but that “air-gap electrodes” are preferable for the analysis of samples which wet or otherwise impair the performance of membranes because the probe does not come into direct contact with the sample.
Although only gas-sensing probes incorporating glass pH electrodes have so far been considered, in fact it is possible to use other ion-selective electrodes as the basis of practical sensors. For example, as shown in Table 1, gas-sensing membrane probes for hydrogen sulfide and hydrogen fluoride are based on sulfide and fluoride ion-selective electrodes, respectively. Probes for these two gases could be based on pH electrodes instead but, as Table 1 indicates, in each case the internal electrolyte used with the preferred ion-selective electrode will effectively prevent interference by other volatile acidic or basic species in the sample, thus significantly enhancing selectivity. Table 1 lists the basic functional details of the best-known gas-sensing membrane probes; in principle, however, probes suitable for the determination of many other gases are feasible, and Ross et al.5 have suggested several possibilities.
Whatever type of ion-selective electrode is used, it is clearly necessary that its configuration should be such as to allow a satisfactory thin film of internal electrolyte to be formed on its ion-selective membrane; in practice, this means that the sensing tip of the electrode will probably be flat or slightly convex. Thus, only a thin film of electrolyte will remain when the sensing tip is pressed against the gas-permeable membrane, minimizing the volume of solution which must e...
Table of contents
- Cover
- Half title
- Title Page
- Copyright PAge
- Preface
- The Editor
- Contributors
- Content Page
- Chapter 1 Gas-Sensing Probes
- Chapter 2 Enzyme Electrodes
- Chapter 3 Ion-Selective Electrodes in Medicine and Medical Research
- Chapter 4 Analytical Methods Involving Ion-Selective Electrodes (Including Flow Methods
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Ion Selective Electrode Method by A.K. Covington in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Biology. We have over one million books available in our catalogue for you to explore.